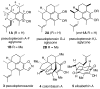
Ethylene in organic synthesis. Repetitive hydrovinylation of alkenes for highly enantioselective syntheses of pseudopterosins
- PMID: 21449569
- PMCID: PMC3087302
- DOI: 10.1021/ja201321v
Ethylene in organic synthesis. Repetitive hydrovinylation of alkenes for highly enantioselective syntheses of pseudopterosins
Abstract
In this report we highlight the significant potential of ethylene as a reagent for the introduction of a vinyl group with excellent stereoselectivity at three different stages in the synthesis of a broad class of natural products, best exemplified by syntheses of pseudopterosins. The late-stage applications of the asymmetric hydrovinylation reaction further illustrate the compatibility of the catalyst with complex functional groups. We also show that, depending on the choice of the catalyst, it is possible to either enhance or even completely reverse the inherent diastereoselectivity in the reactions of advanced chiral intermediates. This should enable the synthesis of diastereomeric analogs of several classes of medicinally relevant compounds that are not readily accessible by the existing methods, which depend on 'substrate control' for the installation of many of the chiral centers, especially in molecules of this class.
Figures
References
-
-
For examples of the use of unactivated alkenes including ethylene as reagents in catalytic reactions, see: Jolly PW, Wilke G. Hydrovinylation. In: Cornils B, Herrmann WA, editors. Applied Homogeneous Catalysis with Organometallic Compounds. Vol. 2. VCH; New York: 1996. pp. 1024–1048.RajanBabu TV. Chem Rev. 2003;103:2845.Bower JF, Kim IS, Patman RL, Krische MJ. Angew Chem Int Ed Engl. 2009;48:34.Ho CY, Schleicher KD, Chan CW, Jamison TF. Synlett. 2009:2565.Hess W, Treutwein J, Hilt G. Synthesis. 2008:3537.For recent, representative examples of use of ethylene in fine chemical synthesis: Mori M, Saito N, Tanaka D, Takimoto M, Sato Y. J Am Chem Soc. 2003;125:5606.Ogoshi S, Haba T, Ohashi M. J Am Chem Soc. 2009;131:10350.Smith CR, RajanBabu TV. Tetrahedron. 2010;66:1102.Sharma RK, RajanBabu TV. J Am Chem Soc. 2010;132:3295.Matsubara R, Jamison TF. J Am Chem Soc. 2010;132:6880.An outstanding example of iterative use of an asymmetric C-C bond-forming reaction in natural product synthesis, see: Han SB, Hassan A, Kim IS, Krische MJ. J Am Chem Soc. 2010;132:15559.For a review of asymmetric catalysis in complex molecule synthesis, see: Taylor MS, Jacobsen EN. Proc Natl Acad Sci U S A. 2004;101:5368.
-
-
-
Buszek KR, Bixby DL. Tetrahedron Lett. 1995;36:9129.(b) see also ref (9).
-
-
- Look SA, Fenical W, Jacobs RS, Clardy J. Proc Natl Acad Sci U S A. 1986;83:6238. - PMC - PubMed
- Rodríguez AD, Ramírez C, Rodríguez II, González E. Org Lett. 1999;1:527. - PubMed
- Rodríguez AD, Ramírez C. Org Lett. 2000;2:507. - PubMed
- Rodríguez AD, Ramírez C, Rodríguez II, Barnes CL. J Org Chem. 2000;65:1390. - PubMed
- Rodríguez AD, González E, Huang SD. J Org Chem. 1998;63:7083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources