Enantioconvergent hydroboration of a racemic allene: enantioselective synthesis of (E)-δ-stannyl-anti-homoallylic alcohols via aldehyde crotylboration
- PMID: 21449594
- PMCID: PMC3086366
- DOI: 10.1021/ja2010187
Enantioconvergent hydroboration of a racemic allene: enantioselective synthesis of (E)-δ-stannyl-anti-homoallylic alcohols via aldehyde crotylboration
Abstract
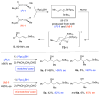
The enantioconvergent hydroboration of racemic allenylstannane (±)-1 with ((d)Ipc)(2)BH converts both enantiomers of (±)-1 into the enantioenriched crotylborane (S)-E-3. Subsequent crotylboration of aldehydes with (S)-E-3 provides (E)-stannyl-homoallylic alcohols 5 in good yields and with excellent enantioselectivity.
Figures
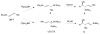


Similar articles
-
Enantioselective synthesis of (E)-δ-silyl-anti-homoallylic alcohols via an enantiodivergent hydroboration-crotylboration reaction of a racemic allenylsilane.Org Lett. 2013 Apr 5;15(7):1662-5. doi: 10.1021/ol4004405. Epub 2013 Mar 27. Org Lett. 2013. PMID: 23534391 Free PMC article.
-
Enantioselective synthesis of (E)-delta-stannyl homoallylic alcohols via aldehyde allylboration using alpha-stannylallylboranes generated by allene hydroboration followed by a highly diastereoselective 1,3-boratropic shift.J Am Chem Soc. 2010 Jun 16;132(23):7881-3. doi: 10.1021/ja103041u. J Am Chem Soc. 2010. PMID: 20496899 Free PMC article.
-
Thermodynamic control of 1,3-boratropic shifts of α- and γ-stannyl-substituted allylboranes: hyperconjugation outweighs steric effects.Org Lett. 2011 Mar 18;13(6):1478-81. doi: 10.1021/ol2001599. Epub 2011 Feb 16. Org Lett. 2011. PMID: 21323387 Free PMC article.
-
Enantioselective synthesis of 1,5-anti- and 1,5-syn-diols using a highly diastereoselective one-pot double allylboration reaction sequence.J Am Chem Soc. 2002 Nov 20;124(46):13644-5. doi: 10.1021/ja028055j. J Am Chem Soc. 2002. PMID: 12431072
-
Tandem reactions for streamlining synthesis: enantio- and diastereoselective one-pot generation of functionalized epoxy alcohols.Acc Chem Res. 2008 Aug;41(8):883-93. doi: 10.1021/ar800006h. Acc Chem Res. 2008. PMID: 18710197 Free PMC article. Review.
Cited by
-
Catalytic asymmetric transformations of racemic α-borylmethyl-(E)-crotylboronate via kinetic resolution or enantioconvergent reaction pathways.Chem Sci. 2021 Sep 20;12(40):13398-13403. doi: 10.1039/d1sc04047b. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777758 Free PMC article.
-
Enantioselective synthesis of (+)-crocacin C. An example of a highly challenging mismatched double asymmetric δ-stannylcrotylboration reaction.Org Lett. 2012 Apr 6;14(7):1880-3. doi: 10.1021/ol300476f. Epub 2012 Mar 12. Org Lett. 2012. PMID: 22409510 Free PMC article.
-
Enantioselective synthesis of (E)-δ-silyl-anti-homoallylic alcohols via an enantiodivergent hydroboration-crotylboration reaction of a racemic allenylsilane.Org Lett. 2013 Apr 5;15(7):1662-5. doi: 10.1021/ol4004405. Epub 2013 Mar 27. Org Lett. 2013. PMID: 23534391 Free PMC article.
-
Ligand-controlled cobalt-catalyzed regiodivergent hydroboration of aryl,alkyl-disubstituted internal allenes.Chem Sci. 2020 Feb 5;11(10):2783-2789. doi: 10.1039/c9sc06136c. Chem Sci. 2020. PMID: 34084338 Free PMC article.
-
(Z)-α-Boryl-crotylboron reagents via Z-selective alkene isomerization and application to stereoselective syntheses of (E)-δ-boryl-syn-homoallylic alcohols.Chem Sci. 2019 Feb 26;10(12):3637-3642. doi: 10.1039/c9sc00226j. eCollection 2019 Mar 28. Chem Sci. 2019. PMID: 30996958 Free PMC article.
References
-
- Jacobsen EN, Pfaltz A, Yamamoto H. Comprehensive Asymmetric Catalysis. I-III. Springer; Berlin: 1999.
- Ojima I. Catalytic Asymmetric Synthesis. 2. Wiley/VCH; New York: 2000.
-
- Kagan HB, Fiaud JC. In: Topics in Stereochemistry. Allinger AL, Eliel E, editors. Vol. 18. Wiley; New York: 1988. p. 249.
- Vedejs E, Jure M. Angew Chem, Int Ed. 2005;44:3974. - PubMed
-
- Noyori R, Tokunaga M, Kitamura M. Bull Chem Soc Jpn. 1995;68:36.
- Huerta FF, Minidis ABE, Backvall JE. Chem Soc Rev. 2001:321.
- Trost BM, Fandrick DR. Aldrichimica Acta. 2007;40:59.
- Pellissier H. Tetrahedron. 2008;64:1563.
-
-
Early examples of the enantioconvergent reactions of racemic allenes were reported by Bergman and co-workers: Sweeney ZK, Salsman JL, Andersen RA, Bergman RG. Angew Chem, Int Ed. 2000;39:2339.However, the products of these reactions were not used in subsequent enantioselective transformations, as is the case with the chemistry reported herein. (b) An example of dynamic kinetic resolution of racemic allenes was reported by Widenhoefer and co-workers: Zhang Z, Bender CF, Widenhoefer RA. J Am Chem Soc. 2007;129:14148.One recent example of the enantioconvergent Cu(I)-mediated borylation of a racemic cyclic allylic ether was reported by Ito and co-workers: Ito H, Kunii S, Sawamura M. Nature Chem. 2010;2:972.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

