Artificial niche combining elastomeric substrate and platelets guides vascular differentiation of bone marrow mononuclear cells
- PMID: 21449713
- PMCID: PMC3142637
- DOI: 10.1089/ten.TEA.2010.0550
Artificial niche combining elastomeric substrate and platelets guides vascular differentiation of bone marrow mononuclear cells
Abstract
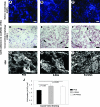
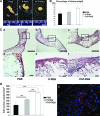
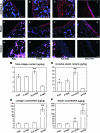
Bone marrow-derived progenitor cells are promising cell sources for vascular tissue engineering. However, conventional bone marrow mesenchymal stem cell expansion and induction strategies require plating on tissue culture plastic, a stiff substrate that may itself influence cell differentiation. Direct scaffold seeding avoids plating on plastic; to the best of our knowledge, there is no report of any scaffold that induces the differentiation of bone marrow mononuclear cells (BMNCs) to vascular cells in vitro. In this study, we hypothesize that an elastomeric scaffold with adsorbed plasma proteins and platelets will induce differentiation of BMNCs to vascular cells and promote vascular tissue formation by combining soft tissue mechanical properties with platelet-mediated tissue repairing signals. To test our hypothesis, we directly seeded rat primary BMNCs in four types of scaffolds: poly(lactide-co-glycolide), elastomeric poly(glycerol sebacate) (PGS), platelet-poor plasma-coated PGS, and PGS coated by plasma supplemented with platelets. After 21 days of culture, osteochondral differentiation of cells in poly(lactide-co-glycolide) was detected, but most of the adhered cells on the surface of all PGS scaffolds expressed calponin-I and α-smooth muscle actin, suggesting smooth muscle differentiation. Cells in PGS scaffolds also produced significant amount of collagen and elastin. Further, plasma coating improves seeding efficiency, and platelet increases proliferation, the number of differentiated cells, and extracellular matrix content. Thus, the artificial niche composed of platelets, plasma, and PGS is promising for artery tissue engineering using BMNCs.
Figures





References
-
- Mirzaei M. Truswell A.S. Taylor R. Leeder S.R. Coronary heart disease epidemics: not all the same. Heart. 2009;95:740. - PubMed
-
- Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217. - PubMed
-
- L'Heureux N. McAllister T.N. de la Fuente L.M. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451. - PubMed
-
- Matsumura G. Hibino N. Ikada Y. Kurosawa H. Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303. - PubMed
-
- Shin'oka T. Matsumura G. Hibino N. Naito Y. Watanabe M. Konuma T., et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

