hERG subunit composition determines differential drug sensitivity
- PMID: 21449979
- PMCID: PMC3188906
- DOI: 10.1111/j.1476-5381.2011.01378.x
hERG subunit composition determines differential drug sensitivity
Abstract
Background and purpose: The majority of human ether-a-go-go-related gene (hERG) screens aiming to minimize the risk of drug-induced long QT syndrome have been conducted using heterologous systems expressing the hERG 1a subunit, although both hERG 1a and 1b subunits contribute to the K+ channels producing the repolarizing current I(Kr) . We tested a range of compounds selected for their diversity to determine whether hERG 1a and 1a/1b channels exhibit different sensitivities that may influence safety margins or contribute to a stratified risk analysis.
Experimental approach: We used the IonWorks™ plate-based electrophysiology device to compare sensitivity of hERG 1a and 1a/1b channels stably expressed in HEK293 cells to 50 compounds previously shown to target hERG channels. Potency was determined as IC₅₀ values (µM) obtained from non-cumulative, eight-point concentration-effect curves of normalized data, fitted to the Hill equation. To minimize possible sources of variability, compound potency was assessed using test plates arranged in alternating columns of cells expressing hERG 1a and 1a/1b.
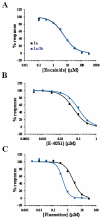
Key results: Although the potency of most compounds was similar for the two targets, some surprising differences were observed. Fluoxetine (Prozac) was more potent at blocking hERG 1a/1b than 1a channels, yielding a corresponding reduction in the safety margin. In contrast, E-4031 was a more potent blocker of hERG 1a compared with 1a/1b channels, as previously reported, as was dofetilide, another high-affinity blocker.
Conclusions and implications: The current assays may underestimate the risk of some drugs to cause torsades de pointes arrhythmia, and overestimate the risk of others.
© 2011 AstraZeneca. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
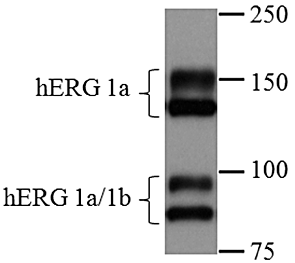


 ) or 1a/1b channels (
) or 1a/1b channels ( ) measured using IonWorks™. The IC50 values for these compounds are shown in Table 1. Although encainide exhibited similar potency for 1a and 1a/1b, E-4031 and fluoxetine exhibited greater potency for 1a (as previously reported by Sale et al., 2008) and 1a/1b respectively.
) measured using IonWorks™. The IC50 values for these compounds are shown in Table 1. Although encainide exhibited similar potency for 1a and 1a/1b, E-4031 and fluoxetine exhibited greater potency for 1a (as previously reported by Sale et al., 2008) and 1a/1b respectively.
References
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Bridgland-Taylor MH, Hargreaves AC, Easter A, Orme A, Henthorn DC, Ding M, et al. Optimisation and validation of a medium-throughput electrophysiology-based hERG assay using IonWork™ HT. J Pharmacol Toxicol Methods. 2006;54:189–199. - PubMed
-
- Ficker E, Jarolimek W, Kiehn J, Baumann A, Brown AM. Molecular determinants of dofetilide block of HERG K+ channels. Circ Res. 1998;82:386–395. - PubMed
-
- Ficker E, Jarolimek W, Brown AM. Molecular determinants of inactivation and dofetilide block in ether a-go-go (EAG) channels and EAG-related K+ channels. Mol Pharmacol. 2001;60:1343–1348. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

