A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury
- PMID: 21449982
- PMCID: PMC3423243
- DOI: 10.1111/j.1476-5381.2011.01381.x
A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury
Abstract
Background and purpose: Cannabinoid CB(2) receptor activation has been reported to attenuate myocardial, cerebral and hepatic ischaemia-reperfusion (I/R) injury.
Experimental approach: We have investigated the effects of a novel CB(2) receptor agonist ((1S,4R)-2-(2,6-dimethoxy-4-(2-methyloctan-2-yl)phenyl)-7,7-dimethylbicyclo[2.2.1]hept-2-en-1-yl)methanol (HU-910) on liver injury induced by 1 h of ischaemia followed by 2, 6 or 24 h of reperfusion, using a well-established mouse model of segmental hepatic I/R.
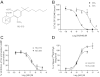
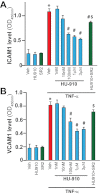
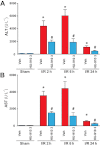
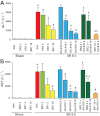
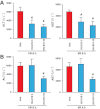
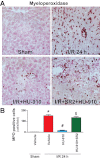
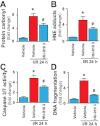
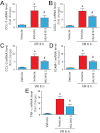
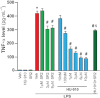
Key results: Displacement of [(3) H]CP55940 by HU-910 from specific binding sites in CHO cell membranes transfected with human CB(2) or CB(1) receptors (hCB(1/2) ) yielded K(i) values of 6 nM and 1.4 µM respectively. HU-910 inhibited forskolin-stimulated cyclic AMP production by hCB(2) CHO cells (EC(50) = 162 nM) and yielded EC(50) of 26.4 nM in [(35) S]GTPγS binding assays using hCB(2) expressing CHO membranes. HU-910 given before ischaemia significantly attenuated levels of I/R-induced hepatic pro-inflammatory chemokines (CCL3 and CXCL2), TNF-α, inter-cellular adhesion molecule-1, neutrophil infiltration, oxidative stress and cell death. Some of the beneficial effect of HU-910 also persisted when given at the beginning of the reperfusion or 1 h after the ischaemic episode. Furthermore, HU-910 attenuated the bacterial endotoxin-triggered TNF-α production in isolated Kupffer cells and expression of adhesion molecules in primary human liver sinusoidal endothelial cells stimulated with TNF-α. Pretreatment with a CB(2) receptor antagonist attenuated the protective effects of HU-910, while pretreatment with a CB(1) antagonist tended to enhance them.
Conclusion and implications: HU-910 is a potent CB(2) receptor agonist which may exert protective effects in various diseases associated with inflammation and tissue injury.
Linked articles: This article is part of a themed section on Cannabinoids in Biology and Medicine. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.165.issue-8. To view Part I of Cannabinoids in Biology and Medicine visit http://dx.doi.org/10.1111/bph.2011.163.issue-7.
Published 2011. This article is a U.S. Government work and is in the public domain in the USA.
Figures





References
-
- Caraceni P, Pertosa AM, Giannone F, Domenicali M, Grattagliano I, Principe A, et al. Antagonism of the cannabinoid CB-1 receptor protects rat liver against ischaemia-reperfusion injury complicated by endotoxaemia. Gut. 2009;58:1135–1143. - PubMed
-
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

