A study protocol for the evaluation of occupational mutagenic/carcinogenic risks in subjects exposed to antineoplastic drugs: a multicentric project
- PMID: 21450074
- PMCID: PMC3074546
- DOI: 10.1186/1471-2458-11-195
A study protocol for the evaluation of occupational mutagenic/carcinogenic risks in subjects exposed to antineoplastic drugs: a multicentric project
Abstract
Background: Some industrial hygiene studies have assessed occupational exposure to antineoplastic drugs; other epidemiological investigations have detected various toxicological effects in exposure groups labeled with the job title. In no research has the same population been studied both environmentally and epidemiologically. The protocol of the epidemiological study presented here uses an integrated environmental and biological monitoring approach. The aim is to assess in hospital nurses preparing and/or administering therapy to cancer patients the current level of occupational exposure to antineoplastic drugs, DNA and chromosome damage as cancer predictive effects, and the association between the two.
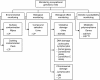
Methods/design: About 80 healthy non-smoking female nurses, who job it is to prepare or handle antineoplastic drugs, and a reference group of about 80 healthy non-smoking female nurses not occupationally exposed to chemicals will be examined simultaneously in a cross-sectional study. All the workers will be recruited from five hospitals in northern and central Italy after their informed consent has been obtained.Evaluation of surface contamination and dermal exposure to antineoplastic drugs will be assessed by determining cyclophosphamide on selected surfaces (wipes) and on the exposed nurses' clothes (pads). The concentration of unmetabolized cyclophosphamide as a biomarker of internal dose will be measured in end-shift urine samples from exposed nurses. Biomarkers of effect and susceptibility will be assessed in exposed and unexposed nurses: urinary concentration of 8-hydroxy-2-deoxyguanosine; DNA damage detected using the single-cell microgel electrophoresis (comet) assay in peripheral white blood cells; micronuclei and chromosome aberrations in peripheral blood lymphocytes. Genetic polymorphisms for enzymes involved in metabolic detoxification (i.e. glutathione S-transferases) will also be analysed.Using standardized questionnaires, occupational exposure will be determined in exposed nurses only, whereas potential confounders (medicine consumption, lifestyle habits, diet and other non-occupational exposures) will be assessed in both groups of hospital workers.Statistical analysis will be performed to ascertain the association between occupational exposure to antineoplastic drugs and biomarkers of DNA and chromosome damage, after taking into account the effects of individual genetic susceptibility, and the presence of confounding exposures.
Discussion: The findings of the study will be useful in updating prevention procedures for handling antineoplastic drugs.
Figures
References
-
- Kiffmeyer T, Hadtstein C. Handling of chemotherapeutic drugs in the OR: hazards and safety considerations. Cancer treatment and research. 2007;134:275–290. - PubMed
-
- Turci R, Sottani C, Spagnoli G, Minoia C. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agents: a review of analytical methods. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2003;789:169–209. doi: 10.1016/S1570-0232(03)00100-4. - DOI - PubMed
-
- McDevitt JJ, Lees PS, McDiarmid MA. Exposure of hospital pharmacists and nurses to antineoplastic agents. J Occup Med. 1993;35:57–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical