Herpes simplex virus 1 ICP4 forms complexes with TFIID and mediator in virus-infected cells
- PMID: 21450820
- PMCID: PMC3126299
- DOI: 10.1128/JVI.00385-11
Herpes simplex virus 1 ICP4 forms complexes with TFIID and mediator in virus-infected cells
Abstract
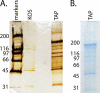
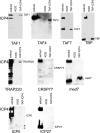
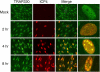
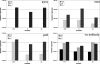
The infected cell polypeptide 4 (ICP4) of herpes simplex virus 1 (HSV-1) is a regulator of viral transcription that is required for productive infection. Since viral genes are transcribed by cellular RNA polymerase II (RNA pol II), ICP4 must interact with components of the pol II machinery to regulate viral gene expression. It has been shown previously that ICP4 interacts with TATA box-binding protein (TBP), TFIIB, and the TBP-associated factor 1 (TAF1) in vitro. In this study, ICP4-containing complexes were isolated from infected cells by tandem affinity purification (TAP). Forty-six proteins that copurified with ICP4 were identified by mass spectrometry. Additional copurifying proteins were identified by Western blot analysis. These included 11 components of TFIID and 4 components of the Mediator complex. The significance of the ICP4-Mediator interaction was further investigated using immunofluorescence and chromatin immunoprecipitation. Mediator was found to colocalize with ICP4 starting at early and continuing into late times of infection. In addition, Mediator was recruited to viral promoters in an ICP4-dependent manner. Taken together, the data suggest that ICP4 interacts with components of TFIID and Mediator in the context of viral infection, and this may explain the broad transactivation properties of ICP4.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

