Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses
- PMID: 21450829
- PMCID: PMC3094968
- DOI: 10.1128/JVI.00313-11
Antibody-dependent cell-mediated viral inhibition emerges after simian immunodeficiency virus SIVmac251 infection of rhesus monkeys coincident with gp140-binding antibodies and is effective against neutralization-resistant viruses
Abstract
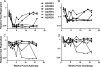
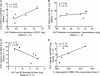
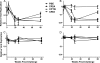
Antibody-dependent cell-mediated viral inhibition (ADCVI) is an attractive target for vaccination because it takes advantage of both the anamnestic properties of an adaptive immune response and the rapid early response characteristics of an innate immune response. Effective utilization of ADCVI in vaccine strategies will depend on an understanding of the natural history of ADCVI during acute and chronic human immunodeficiency virus type 1 (HIV-1) infection. We used the simian immunodeficiency virus (SIV)-infected rhesus monkey as a model to study the kinetics of ADCVI in early infection, the durability of ADCVI through the course of infection, and the effectiveness of ADCVI against viruses with envelope mutations that are known to confer escape from antibody neutralization. We demonstrate the development of ADCVI, capable of inhibiting viral replication 100-fold, within 3 weeks of infection, preceding the development of a comparable-titer neutralizing antibody response by weeks to months. The emergence of ADCVI was temporally associated with the emergence of gp140-binding antibodies, and in most animals, ADCVI persisted through the course of infection. Highly evolved viral envelopes from viruses isolated at late time points following infection that were resistant to plasma neutralization remained susceptible to ADCVI, suggesting that the epitope determinants of neutralization escape are not shared by antibodies that mediate ADCVI. These findings suggest that despite the ability of SIV to mutate and adapt to multiple immunologic pressures during the course of infection, SIV envelope may not escape the binding of autologous antibodies that mediate ADCVI.
Figures

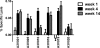

References
-
- Aasa-Chapman M. M., et al. 2004. Development of the antibody response in acute HIV-1 infection. AIDS 18:371–381 - PubMed
-
- Ahmad A., Menezes J. 1996. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 10:258–266 - PubMed
-
- Ahmad R., et al. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227–233 - PubMed
-
- AIDS Vaccine Evaluation Group 022 Protocol Team 2001. Cellular and humoral immune responses to a canarypox vaccine containing human immunodeficiency virus type 1 Env, Gag, and Pro in combination with rgp120. J. Infect. Dis. 183:563–570 - PubMed
-
- Alsmadi O., Tilley S. A. 1998. Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities. J. Virol. 72:286–293 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

