Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool
- PMID: 21450902
- PMCID: PMC3109710
- DOI: 10.1182/blood-2011-01-333476
Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool
Abstract
The stem cell factor (SCF)/Kit system has served as a classic model in deciphering molecular signaling events in the hematopoietic compartment, and Kit expression is a most critical marker for hematopoietic stem cells (HSCs) and progenitors. However, it remains to be elucidated how Kit expression is regulated in HSCs. Herein we report that a cytoplasmic tyrosine phosphatase Shp2, acting downstream of Kit and other RTKs, promotes Kit gene expression, constituting a Kit-Shp2-Kit signaling axis. Inducible ablation of PTPN11/Shp2 resulted in severe cytopenia in BM, spleen, and peripheral blood in mice. Shp2 removal suppressed the functional pool of HSCs/progenitors, and Shp2-deficient HSCs failed to reconstitute lethally irradiated recipients because of defects in homing, self-renewal, and survival. We show that Shp2 regulates coordinately multiple signals involving up-regulation of Kit expression via Gata2. Therefore, this study reveals a critical role of Shp2 in maintenance of a functional HSC/progenitor pool in adult mammals, at least in part through a kinase-phosphatase-kinase cascade.
Figures
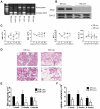


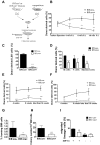
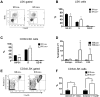

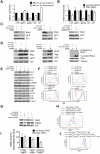
Similar articles
-
Loss of c-Kit and bone marrow failure upon conditional removal of the GATA-2 C-terminal zinc finger domain in adult mice.Eur J Haematol. 2016 Sep;97(3):261-70. doi: 10.1111/ejh.12719. Epub 2016 Jan 14. Eur J Haematol. 2016. PMID: 26660446 Free PMC article.
-
The dynamic interplay between a PTK (Kit) and a PTP (Shp2) in hematopoietic stem and progenitor cells.Cell Cycle. 2011 Jul 15;10(14):2241-2. doi: 10.4161/cc.10.14.15861. Epub 2011 Jul 15. Cell Cycle. 2011. PMID: 21701261 Free PMC article. No abstract available.
-
Role of SHP2 phosphatase in KIT-induced transformation: identification of SHP2 as a druggable target in diseases involving oncogenic KIT.Blood. 2012 Sep 27;120(13):2669-78. doi: 10.1182/blood-2011-08-375873. Epub 2012 Jul 17. Blood. 2012. PMID: 22806893 Free PMC article.
-
Kit and Scl regulation of hematopoietic stem cells.Curr Opin Hematol. 2014 Jul;21(4):256-64. doi: 10.1097/MOH.0000000000000052. Curr Opin Hematol. 2014. PMID: 24857885 Review.
-
Shp2 function in hematopoietic stem cell biology and leukemogenesis.Curr Opin Hematol. 2012 Jul;19(4):273-9. doi: 10.1097/MOH.0b013e328353c6bf. Curr Opin Hematol. 2012. PMID: 22504523 Free PMC article. Review.
Cited by
-
Shp2 promotes metastasis of prostate cancer by attenuating the PAR3/PAR6/aPKC polarity protein complex and enhancing epithelial-to-mesenchymal transition.Oncogene. 2016 Mar 10;35(10):1271-82. doi: 10.1038/onc.2015.184. Epub 2015 Jun 8. Oncogene. 2016. PMID: 26050620
-
SHP2-Mediated Signal Networks in Stem Cell Homeostasis and Dysfunction.Stem Cells Int. 2018 Jun 10;2018:8351374. doi: 10.1155/2018/8351374. eCollection 2018. Stem Cells Int. 2018. PMID: 29983715 Free PMC article. Review.
-
SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells.Oncotarget. 2017 Jun 21;8(32):53518-53530. doi: 10.18632/oncotarget.18591. eCollection 2017 Aug 8. Oncotarget. 2017. PMID: 28881828 Free PMC article.
-
Steamed Panax notoginseng Attenuates Anemia in Mice With Blood Deficiency Syndrome via Regulating Hematopoietic Factors and JAK-STAT Pathway.Front Pharmacol. 2020 Jan 21;10:1578. doi: 10.3389/fphar.2019.01578. eCollection 2019. Front Pharmacol. 2020. PMID: 32038252 Free PMC article.
-
Nonreceptor tyrosine phosphatase Shp2 promotes adipogenesis through inhibition of p38 MAP kinase.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):E79-88. doi: 10.1073/pnas.1213000110. Epub 2012 Dec 10. Proc Natl Acad Sci U S A. 2013. PMID: 23236157 Free PMC article.
References
-
- Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134(11):2001–2006. - PubMed
-
- Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335(6185):88–89. - PubMed
-
- Chui DH, Loyer BV, Russell ES. Steel (Sl) mutation in mice: identification of mutant embryos early in development. Dev Biol. 1976;49(1):300–303. - PubMed
-
- Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

