The tail-elicited tail withdrawal reflex of Aplysia is mediated centrally at tail sensory-motor synapses and exhibits sensitization across multiple temporal domains
- PMID: 21450911
- PMCID: PMC3072776
- DOI: 10.1101/lm.2125311
The tail-elicited tail withdrawal reflex of Aplysia is mediated centrally at tail sensory-motor synapses and exhibits sensitization across multiple temporal domains
Abstract
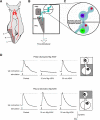
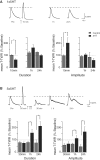
The defensive withdrawal reflexes of Aplysia californica have provided powerful behavioral systems for studying the cellular and molecular basis of memory formation. Among these reflexes the tail-elicited tail withdrawal reflex (T-TWR) has been especially useful. In vitro studies examining the monosynaptic circuit for the T-TWR, the tail sensory-motor (SN-MN) synapses, have identified the induction requirements and molecular basis of different temporal phases of synaptic facilitation that underlie sensitization in this system. They have also permitted more recent studies elucidating the role of synaptic and nuclear signaling during synaptic facilitation. Here we report the development of a novel, compartmentalized semi-intact T-TWR preparation that allows examination of the unique contributions of processing in the SN somatic compartment (the pleural ganglion) and the SN-MN synaptic compartment (the pedal ganglion) during the induction of sensitization. Using this preparation we find that the T-TWR is mediated entirely by central connections in the synaptic compartment. Moreover, the reflex is stably expressed for at least 24 h, and can be modified by tail shocks that induce sensitization across multiple temporal domains, as well as direct application of the modulatory neurotransmitter serotonin. This preparation now provides an experimentally powerful system in which to directly examine the unique and combined roles of synaptic and nuclear signaling in different temporal domains of memory formation.
Figures




Similar articles
-
Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity.J Neurosci. 2002 Mar 15;22(6):2299-312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. J Neurosci. 2002. PMID: 11896169 Free PMC article.
-
The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex.J Neurosci. 1999 Dec 1;19(23):10438-50. doi: 10.1523/JNEUROSCI.19-23-10438.1999. J Neurosci. 1999. PMID: 10575041 Free PMC article.
-
Heterosynaptic facilitation of tail sensory neuron synaptic transmission during habituation in tail-induced tail and siphon withdrawal reflexes of Aplysia.J Neurosci. 1996 Aug 15;16(16):4933-48. doi: 10.1523/JNEUROSCI.16-16-04933.1996. J Neurosci. 1996. PMID: 8756425 Free PMC article.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Insights into a molecular switch that gates sensory neuron synapses during habituation in Aplysia.Neurobiol Learn Mem. 2009 Sep;92(2):155-65. doi: 10.1016/j.nlm.2009.03.006. Epub 2009 Apr 2. Neurobiol Learn Mem. 2009. PMID: 19345275 Free PMC article. Review.
Cited by
-
Role of nitric oxide in the induction of the behavioral and cellular changes produced by a common aversive stimulus in Aplysia.Behav Brain Res. 2019 Mar 15;360:341-353. doi: 10.1016/j.bbr.2018.12.010. Epub 2018 Dec 6. Behav Brain Res. 2019. PMID: 30528940 Free PMC article.
-
Unraveling the complexities of circadian and sleep interactions with memory formation through invertebrate research.Front Syst Neurosci. 2014 Aug 4;8:133. doi: 10.3389/fnsys.2014.00133. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25136297 Free PMC article. Review.
-
Pattern and predictability in memory formation: from molecular mechanisms to clinical relevance.Neurobiol Learn Mem. 2013 Oct;105:117-24. doi: 10.1016/j.nlm.2013.05.003. Epub 2013 May 28. Neurobiol Learn Mem. 2013. PMID: 23727358 Free PMC article. Review.
-
Understanding intellectual disability through RASopathies.J Physiol Paris. 2014 Sep-Dec;108(4-6):232-9. doi: 10.1016/j.jphysparis.2014.05.003. Epub 2014 May 21. J Physiol Paris. 2014. PMID: 24859216 Free PMC article. Review.
-
Distinct Growth Factor Families Are Recruited in Unique Spatiotemporal Domains during Long-Term Memory Formation in Aplysia californica.Neuron. 2015 Jun 3;86(5):1228-39. doi: 10.1016/j.neuron.2015.04.025. Neuron. 2015. PMID: 26050041 Free PMC article.
References
-
- Antonov I, Antonova I, Kandel ER, Hawkins RD 2003. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron 37: 135–147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
