A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers
- PMID: 21451004
- PMCID: PMC3068023
- DOI: 10.1093/infdis/jiq175
A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers
Abstract
Background: A key missing element in the development of a successful human immunodeficiency virus (HIV) vaccine is an immunogen that can generate broadly cross-neutralizing antibodies against primary isolates of the virus.
Methods: This phase 1 clinical trial employed a DNA prime and subunit envelope protein boost in an attempt to generate cellular and humoral immune responses that might be desirable in a protective HIV vaccine. Priming was performed via intramuscular injection with gag and env DNA adsorbed to polylactide coglycolide microspheres, followed by boosting with a recombinant trimeric envelope (Env) glycoprotein delivered in MF59 adjuvant.
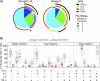
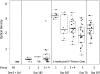
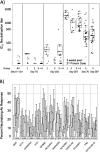
Results: The DNA prime and protein boost were generally safe and well-tolerated. Env-specific CD4(+) cellular responses were generated that were predominantly detected after Env protein boosting. Neutralizing antibody responses against the homologous SF162 viral isolate were remarkably strong and were present in the majority of vaccine recipients, including a strong response against CD4-induced epitopes on gp120. Despite the promising potency of this vaccine approach, neutralization breadth against heterologous tier 2 strains of HIV-1 was minimal.
Conclusions: Potent neutralization against neutralization-sensitive strains of HIV is achievable in humans through a DNA prime, recombinant oligomeric Env protein boost regimen. Eliciting substantial breadth of neutralization remains an elusive goal.
Clinical trials registration: NCT00073216.
© The Author 2011. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved.
Figures



Comment in
-
Clinical evaluation of a soluble trimeric HIV-1 envelope glycoprotein vaccine.Expert Rev Vaccines. 2011 Aug;10(8):1117-20. doi: 10.1586/erv.11.97. Expert Rev Vaccines. 2011. PMID: 21854306
References
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. - PubMed
-
- Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

