Basal ganglia neurons dynamically facilitate exploration during associative learning
- PMID: 21451026
- PMCID: PMC3486636
- DOI: 10.1523/JNEUROSCI.3658-10.2011
Basal ganglia neurons dynamically facilitate exploration during associative learning
Abstract
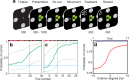
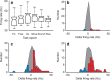
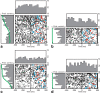
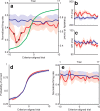
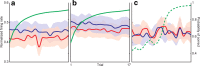
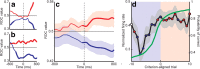
The basal ganglia (BG) appear to play a prominent role in associative learning, the process of pairing external stimuli with rewarding responses. Accumulating evidence suggests that the contributions of various BG components may be described within a reinforcement learning model, in which a broad repertoire of possible responses to environmental stimuli are evaluated before the most profitable one is chosen. The striatum receives diverse cortical inputs, providing a rich source of contextual information about environmental cues. It also receives projections from midbrain dopaminergic neurons, whose phasic activity reflects a reward prediction error signal. These coincident information streams are well suited for evaluating responses and biasing future actions toward the most profitable response. Still lacking in this model is a mechanistic description of how initial response variability is generated. To investigate this question, we recorded the activity of single neurons in the globus pallidus internus (GPi), the primary BG output nucleus, in nonhuman primates (Macaca mulatta) performing a motor associative learning task. A subset (29%) of GPi neurons showed learning-related effects, decreasing firing during the early stages of learning, then returning to higher baseline rates as associations were mastered. On a trial-by-trial basis, lower firing rates predicted exploratory behavior, whereas higher rates predicted an exploitive response. These results suggest that, during associative learning, BG output is initially permissive, allowing exploration of a variety of responses. Once a profitable response is identified, increased GPi activity suppresses alternative responses, sharpening the response profile and encouraging exploitation of the profitable learned behavior.
Figures


References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. - PubMed
-
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. - PubMed
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
