Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury
- PMID: 21451029
- PMCID: PMC3103868
- DOI: 10.1523/JNEUROSCI.5265-10.2011
Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury
Abstract
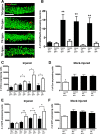
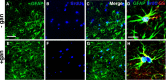
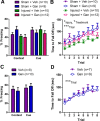
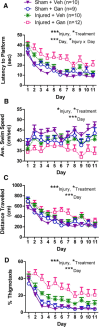
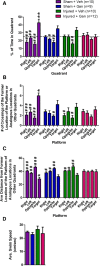
Significant spontaneous recovery occurs after essentially all forms of serious brain injury, although the mechanisms underlying this recovery are unknown. Given that many forms of brain injury such as traumatic brain injury (TBI) induce hippocampal neurogenesis, we investigated whether these newly generated neurons might play a role in recovery. By modeling TBI in transgenic mice, we determined that injury-induced newly generated neurons persisted over time and elaborated extensive dendritic trees that stably incorporated themselves throughout all neuronal layers of the dentate gyrus. When we selectively ablated dividing stem/progenitors at the time of injury with ganciclovir in a nestin-HSV-TK transgenic model, we eliminated injury-induced neurogenesis and subsequently diminished the progenitor pool. Moreover, using hippocampal-specific behavioral tests, we demonstrated that only injured animals with neurogenesis ablated at the time of injury lost the ability to learn spatial memory tasks. These data demonstrate a functional role for adult neurogenesis after brain injury and offer compelling and testable therapeutic options that might enhance recovery.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
