The Wnt5/planar cell polarity pathway regulates axonal development of the Drosophila mushroom body neuron
- PMID: 21451033
- PMCID: PMC6622988
- DOI: 10.1523/JNEUROSCI.0154-11.2011
The Wnt5/planar cell polarity pathway regulates axonal development of the Drosophila mushroom body neuron
Abstract
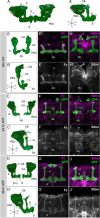
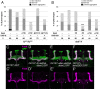
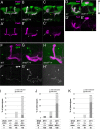
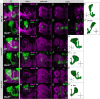
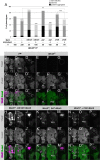
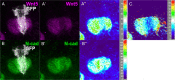
Axonal development is a fundamental process for circuit formation in the nervous system and is dependent on many cellular events, including axon initiation, elongation, guidance, and branching. The molecular mechanisms underlying these events have been well studied, especially for axon guidance. In the presence of a guidance cue, the polarization of a growth cone precedes the turning response, which is one example of the diverse forms of cell polarity. Planar cell polarity (PCP) is another example of cell polarity. Although some PCP genes are required for axonal tract formation in vertebrates, it remains elusive whether these genes participate in a common PCP pathway concertedly. Here, we show that essential PCP signaling components, encoded by frizzled (fz), strabismus (stbm), flamingo (fmi), and dishevelled (dsh), are cooperatively required for axonal targeting and branching of the Drosophila mushroom body (MB) neurons. A detailed analysis of these mutants revealed that these components were required for the correct targeting and bifurcation of axons. In addition, we suggest that Wnt5 functions as a ligand in the PCP pathway in this process. Wnt5 mutants showed similar phenotypes to PCP mutants at the single-cell level and genetically interacted with PCP genes. Wnt5 was broadly expressed in the developing brain. We propose that Wnt5 and the PCP pathway concertedly regulate axonal development of the MB.
Figures

References
-
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. - PubMed
-
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
