An intrinsic neural oscillator in the degenerating mouse retina
- PMID: 21451038
- PMCID: PMC6622979
- DOI: 10.1523/JNEUROSCI.5800-10.2011
An intrinsic neural oscillator in the degenerating mouse retina
Abstract
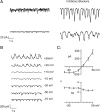
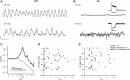
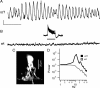
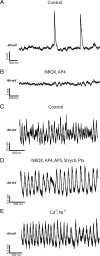
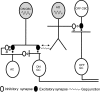
The loss of photoreceptors during retinal degeneration (RD) is known to lead to an increase in basal activity in remnant neural networks. To identify the source of activity, we combined two-photon imaging with patch-clamp techniques to examine the physiological properties of morphologically identified retinal neurons in a mouse model of RD (rd1). Analysis of activity in rd1 ganglion cells revealed sustained oscillatory (∼10 Hz) synaptic activity in ∼30% of all classes of cells. Oscillatory activity persisted after putative inputs from residual photoreceptor, rod bipolar cell, and inhibitory amacrine cell synapses were pharmacologically blocked, suggesting that presynaptic cone bipolar cells were intrinsically active. Examination of presynaptic rd1 ON and OFF bipolar cells indicated that they rested at relatively negative potentials (less than -50 mV). However, in approximately half the cone bipolar cells, low-amplitude membrane oscillation (∼5 mV, ∼10 Hz) were apparent. Such oscillations were also observed in AII amacrine cells. Oscillations in ON cone bipolar and AII amacrine cells exhibited a weak apparent voltage dependence and were resistant to blockade of synaptic receptors, suggesting that, as in wild-type retina, they form an electrically coupled network. In addition, oscillations were insensitive to blockers of voltage-gated Ca(2+) channels (0.5 mm Cd(2+) and 0.5 mm Ni(2+)), ruling out known mechanisms that underlie oscillatory behavior in bipolar cells. Together, these results indicate that an electrically coupled network of ON cone bipolar/AII amacrine cells constitutes an intrinsic oscillator in the rd1 retina that is likely to drive synaptic activity in downstream circuits.
Figures

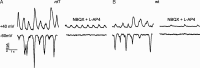



References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
