Different fear states engage distinct networks within the intercalated cell clusters of the amygdala
- PMID: 21451049
- PMCID: PMC6622967
- DOI: 10.1523/JNEUROSCI.6100-10.2011
Different fear states engage distinct networks within the intercalated cell clusters of the amygdala
Abstract
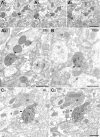
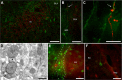
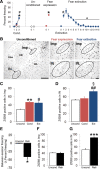
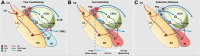
Although extinction-based therapies are among the most effective treatments for anxiety disorders, the neural bases of fear extinction remain still essentially unclear. Recent evidence suggests that the intercalated cell masses of the amygdala (ITCs) are critical structures for fear extinction. However, the neuronal organization of ITCs and how distinct clusters contribute to different fear states are still entirely unknown. Here, by combining whole-cell patch-clamp recordings and biocytin labeling with full anatomical reconstruction of the filled neurons and ultrastructural analysis of their synaptic contacts, we have elucidated the cellular organization and efferent connections of one of the main ITC clusters in mice. Our data showed an unexpected heterogeneity in the axonal pattern of medial paracapsular ITC (Imp) neurons and the presence of three distinct neuronal subtypes. Functionally, we observed that the Imp was preferentially activated during fear expression, whereas extinction training and extinction retrieval activated the main ITC nucleus (IN), as measured by quantifying Zif268 expression. This can be explained by the IPSPs evoked in the IN after Imp stimulation, most likely through the GABAergic monosynaptic innervation of IN neurons by one subtype of Imp cells, namely the medial capsular-projecting (MCp)-Imp neurons. MCp-Imp neurons also target large ITC cells that surround ITC clusters and express the metabotropic glutamate receptor 1α. These findings reveal a distinctive participation of ITC clusters to different fear states and the underlying anatomical circuitries, hence shedding new light on ITC networks and providing a novel framework to elucidate their role in fear expression and extinction.
Figures





References
-
- Alheid G, de Olmos J, Beltramino C. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. Sydney: Academic; 1995. pp. 495–578.
-
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. - PubMed
-
- Ausubel FM. Short protocols in molecular biology. New York: Wiley; 1992.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
