Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses
- PMID: 21451586
- PMCID: PMC3160684
- DOI: 10.1038/ismej.2011.26
Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses
Abstract
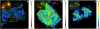
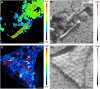
Many diatoms that inhabit low-nutrient waters of the open ocean live in close association with cyanobacteria. Some of these associations are believed to be mutualistic, where N(2)-fixing cyanobacterial symbionts provide N for the diatoms. Rates of N(2) fixation by symbiotic cyanobacteria and the N transfer to their diatom partners were measured using a high-resolution nanometer scale secondary ion mass spectrometry approach in natural populations. Cell-specific rates of N(2) fixation (1.15-71.5 fmol N per cell h(-1)) were similar amongst the symbioses and rapid transfer (within 30 min) of fixed N was also measured. Similar growth rates for the diatoms and their symbionts were determined and the symbiotic growth rates were higher than those estimated for free-living cells. The N(2) fixation rates estimated for Richelia and Calothrix symbionts were 171-420 times higher when the cells were symbiotic compared with the rates estimated for the cells living freely. When combined, the latter two results suggest that the diatom partners influence the growth and metabolism of their cyanobacterial symbionts. We estimated that Richelia fix 81-744% more N than needed for their own growth and up to 97.3% of the fixed N is transferred to the diatom partners. This study provides new information on the mechanisms controlling N input into the open ocean by symbiotic microorganisms, which are widespread and important for oceanic primary production. Further, this is the first demonstration of N transfer from an N(2) fixer to a unicellular partner. These symbioses are important models for molecular regulation and nutrient exchange in symbiotic systems.
Figures

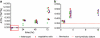
References
-
- Carpenter EJ, Capone DG.2008Nitrogen fixation in the marine environmentIn: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ (eds)Nitrogen in the Marine Environment Academic Press: London; 141–198.
-
- Carpenter EJ, Janson S. Intracellular symbionts in the marine diatom Climacodium frauenfeldianum Grunow. J Phycol. 2000;36:540–544. - PubMed
-
- Carpenter EJ, Montoya JP, Burns JA, Mulholland M, Subramaniam A, Capone DG. Extensive bloom of a N2-fixing diatom/cyanobacterial association in the Tropical Atlantic Ocean. Mar Ecol Prog Ser. 1999;185:273–283.
-
- Clode PL, Stern RA, Marshall AT. Subcellular imaging of isotopically labeled carbon compounds in a biological sample by ion microprobe (NanoSIMS) Microsc Res Tech. 2007;70:220–229. - PubMed
-
- Codispoti L. Is the ocean losing fixed nitrogen. Nature. 1995;376:724.

