Inductive Effects on the Energetics of Prolyl Peptide Bond Isomerization: Implications for Collagen Folding and Stability
- PMID: 21451735
- PMCID: PMC3065073
- DOI: 10.1021/ja9623119
Inductive Effects on the Energetics of Prolyl Peptide Bond Isomerization: Implications for Collagen Folding and Stability
Abstract
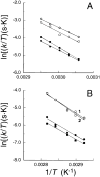
The hydroxylation of proline residues in collagen enhances the stability of the collagen triple helix. Previous X-ray diffraction analyses had demonstrated that the presence of an electron-withdrawing substituent on the pyrrolidine ring of proline residues has significant structural consequences [Panasik, N., Jr.; Eberhardt, E. S.; Edison, A. S.; Powell, D. R.; Raines, R. T. Int. J. Pept. Protein Res.1994, 44, 262-269]. Here, NMR and FTIR spectroscopy were used to ascertain kinetic and thermodynamic properties of N-acetyl-[β,γ-(13)C]D,L-proline methylester (1); N-acetyl-4(R)-hydroxy-L-proline [(13)C]methylester (2); and N-acetyl-4(R)-fluoro-L-proline methylester (3). The pK(a)'s of the nitrogen atom in the parent amino acids decrease in the order: proline (10.8) > 4(R)-hydroxy-L-proline (9.68) > 4(R)-fluoro-L-proline (9.23). In water or dioxane, amide I vibrational modes decrease in the order: 1 > 2 > 3. At 37 °C in dioxane, the rate constants for amide bond isomerization are greater for 3 than 1. Each of these results is consistent with the traditional picture of amide resonance coupled with an inductive effect that results in a higher bond order in the amide C=O bond and a lower bond order in the amide C-N bond. Further, at 37 °C in water or dioxane equilibrium concentrations of the trans isomer increase in the order: 1 < 2 < 3. Inductive effects may therefore have a significant impact on the folding and stability of collagen, which has a preponderance of hydroxyproline residues, all with peptide bonds in the trans conformation.
Figures


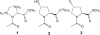
References
-
- Ramachandran GN, Reddi AH, editors. Biochemistry of Collagen. Plenum Press; New York: 1976.
-
- Nimni ME. Collagen. CRC Press; Boca Raton, Fl: 1988.
-
- Rich A, Crick FHC. J Mol Biol. 1961;3:483–506. - PubMed
-
- Baldwin CT, Constanteu CD, Dumars KW, Prockop DJ. J Biol Chem. 1989;264:3002–3006. - PubMed
-
- Kadler K. Protein Profile. 1994. pp. 519–638. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
