The role of liver-directed surgery in patients with hepatic metastasis from a gynecologic primary carcinoma
- PMID: 21452068
- PMCID: PMC3568526
- DOI: 10.1007/s00268-011-1074-y
The role of liver-directed surgery in patients with hepatic metastasis from a gynecologic primary carcinoma
Abstract
Background: The management of patients with liver metastasis from a gynecologic carcinoma remains controversial, as there is currently little data available. We sought to determine the safety and efficacy of liver-directed surgery for hepatic metastasis from gynecologic primaries.
Methods: Between 1990 and 2010, 87 patients with biopsy-proven liver metastasis from a gynecologic carcinoma were identified from an institutional hepatobiliary database. Fifty-two (60%) patients who underwent hepatic surgery for their liver disease and 35 (40%) patients who underwent biopsy only were matched for age, primary tumor characteristics, and hepatic tumor burden. Clinicopathologic, operative, and outcome data were collected and analyzed.
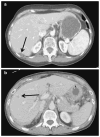
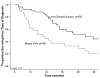
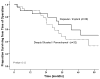
Results: Of the 87 patients, 30 (34%) presented with synchronous metastasis. The majority of patients had multiple hepatic tumors (63%), with a median size of the largest lesion being 2.5 cm. Of those patients who underwent liver surgery (n=52), most underwent a minor hepatic resection (n=44; 85%), while 29 (56%) patients underwent concurrent lymphadenectomy and 45 (87%) patients underwent simultaneous peritoneal debulking. Postoperative morbidity and mortality were 37% and 0%, respectively. Median survival from time of diagnosis was 53 months for patients who underwent liver-directed surgery compared with 21 months for patients who underwent biopsy alone (n=35) (p=0.01). Among those patients who underwent liver-directed surgery, 5-year survival following hepatic resection was 41%.
Conclusions: Hepatic surgery for liver metastasis from gynecologic cancer can be performed safely. Liver surgery may be associated with prolonged survival in a subset of patients with hepatic metastasis from gynecologic primaries and therefore should be considered in carefully selected patients.
Figures

References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. - PubMed
-
- Dauplat J, Hacker NF, Nieberg RK, et al. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987;60(7):1561–1566. - PubMed
-
- Bonnefoi H, A’Hern RP, Fisher C, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol. 1999;17(3):767–775. - PubMed
-
- Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003;13(2):125–129. - PubMed
-
- Rose PG, Piver MS, Tsukada Y, Lau TS. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989;64(7):1508–1513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

