Conditional knockout of protein O-mannosyltransferase 2 reveals tissue-specific roles of O-mannosyl glycosylation in brain development
- PMID: 21452199
- PMCID: PMC3634366
- DOI: 10.1002/cne.22572
Conditional knockout of protein O-mannosyltransferase 2 reveals tissue-specific roles of O-mannosyl glycosylation in brain development
Abstract
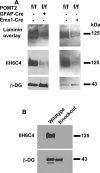
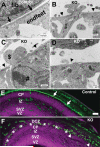
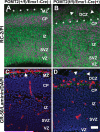
The meninges produce essential signaling molecules and major protein components of the pial basement membrane during normal brain development. Disruptions in the pial basement membrane underlie neural ectopia seen in those congenital muscular dystrophies (CMDs) caused by mutations in genes involved in O-mannosyl glycosylation. In mammals, biosynthesis of O-mannosyl glycans is initiated by a complex of mutually indispensable protein O-mannosyltransferases 1 and 2 (POMT1 and 2). To study the roles of O-mannosylation in brain development we generated a conditional allele of POMT2. POMT2 nulllizygosity resulted in embryonic lethality because of a defective Reichert's membrane. Brain-specific deletion of POMT2 resulted in hypoglycosylation of α-dystroglycan (DG) and abolished laminin binding activity. The effect of POMT2 deletion on brain development was dependent on timing, as earlier deletion resulted in more severe phenotypes. Multiple brain malformations including overmigration of neocortical neurons and migration failure of granule cells in the cerebellum were observed. Immunofluorescence staining and transmission electron microscopy revealed that these migration defects were closely associated with disruptions in the pial basement membrane. Interestingly, POMT2 deletion in the meninges (and blood vessels) did not disrupt the development of the neocortex. Thus, normal brain development requires protein O-mannosylation activity in neural tissue but not the meninges. These results suggest that gene therapy should be directed to the neural tissue instead of the meninges.
Copyright © 2010 Wiley-Liss, Inc.
Figures




Similar articles
-
Differential glycosylation of α-dystroglycan and proteins other than α-dystroglycan by like-glycosyltransferase.Glycobiology. 2012 Feb;22(2):235-47. doi: 10.1093/glycob/cwr131. Epub 2011 Sep 19. Glycobiology. 2012. PMID: 21930648 Free PMC article.
-
Sarcolemma resilience and skeletal muscle health require O-mannosylation of dystroglycan.Skelet Muscle. 2025 Jan 9;15(1):1. doi: 10.1186/s13395-024-00370-2. Skelet Muscle. 2025. PMID: 39789642 Free PMC article.
-
POMT1 is essential for protein O-mannosylation in mammals.Methods Enzymol. 2010;479:323-42. doi: 10.1016/S0076-6879(10)79018-2. Methods Enzymol. 2010. PMID: 20816174
-
Protein O-mannosylation in animal development and physiology: from human disorders to Drosophila phenotypes.Semin Cell Dev Biol. 2010 Aug;21(6):622-30. doi: 10.1016/j.semcdb.2010.03.010. Epub 2010 Apr 1. Semin Cell Dev Biol. 2010. PMID: 20362685 Free PMC article. Review.
-
Mammalian O-mannosylation: unsolved questions of structure/function.Curr Opin Struct Biol. 2011 Oct;21(5):603-9. doi: 10.1016/j.sbi.2011.09.001. Epub 2011 Sep 22. Curr Opin Struct Biol. 2011. PMID: 21945038 Free PMC article. Review.
Cited by
-
Clinical and Molecular Spectrum of Muscular Dystrophies (MDs) with Intellectual Disability (ID): a Comprehensive Overview.J Mol Neurosci. 2022 Jan;72(1):9-23. doi: 10.1007/s12031-021-01933-4. Epub 2021 Nov 2. J Mol Neurosci. 2022. PMID: 34727324 Review.
-
Developmental and mechanical roles of Reichert's membrane in mouse embryos.Philos Trans R Soc Lond B Biol Sci. 2022 Dec 5;377(1865):20210257. doi: 10.1098/rstb.2021.0257. Epub 2022 Oct 17. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 36252218 Free PMC article. Review.
-
Protein O-Mannosylation in the Murine Brain: Occurrence of Mono-O-Mannosyl Glycans and Identification of New Substrates.PLoS One. 2016 Nov 3;11(11):e0166119. doi: 10.1371/journal.pone.0166119. eCollection 2016. PLoS One. 2016. PMID: 27812179 Free PMC article.
-
Integrative mechanisms of oriented neuronal migration in the developing brain.Annu Rev Cell Dev Biol. 2013;29:299-353. doi: 10.1146/annurev-cellbio-101512-122400. Epub 2013 Aug 7. Annu Rev Cell Dev Biol. 2013. PMID: 23937349 Free PMC article. Review.
-
Differential glycosylation of α-dystroglycan and proteins other than α-dystroglycan by like-glycosyltransferase.Glycobiology. 2012 Feb;22(2):235-47. doi: 10.1093/glycob/cwr131. Epub 2011 Sep 19. Glycobiology. 2012. PMID: 21930648 Free PMC article.
References
-
- Akasaka-Manya K, Manya H, Nakajima A, Kawakita M, Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J Biol Chem. 2006;281:19339–193345. - PubMed
-
- Beltran-Valero de BD, Currier S, Steinbrecher A, Celli J, van BE, van der ZB, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van BH, Brunner HG. Mutations in the O-mannosyl-transferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. - PMC - PubMed
-
- Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, Voit T, Sewry CA, Guicheney P, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001a;69:1198–1209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

