Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward γ-aminobutyric acidergic modulators of entorhinal-hippocampal interplay
- PMID: 21452204
- PMCID: PMC4015525
- DOI: 10.1002/cne.22577
Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward γ-aminobutyric acidergic modulators of entorhinal-hippocampal interplay
Abstract
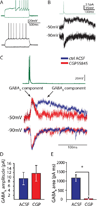
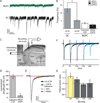
Feed-forward inhibition from molecular layer interneurons onto granule cells (GCs) in the dentate gyrus is thought to have major effects regulating entorhinal-hippocampal interactions, but the precise identity, properties, and functional connectivity of the GABAergic cells in the molecular layer are not well understood. We used single and paired intracellular patch clamp recordings from post-hoc-identified cells in acute rat hippocampal slices and identified a subpopulation of molecular layer interneurons that expressed immunocytochemical markers present in members of the neurogliaform cell (NGFC) class. Single NGFCs displayed small dendritic trees, and their characteristically dense axonal arborizations covered significant portions of the outer and middle one-thirds of the molecular layer, with frequent axonal projections across the fissure into the CA1 and subicular regions. Typical NGFCs exhibited a late firing pattern with a ramp in membrane potential prior to firing action potentials, and single spikes in NGFCs evoked biphasic, prolonged GABA(A) and GABA(B) postsynaptic responses in GCs. In addition to providing dendritic GABAergic inputs to GCs, NGFCs also formed chemical synapses and gap junctions with various molecular layer interneurons, including other NGFCs. NGFCs received low-frequency spontaneous synaptic events, and stimulation of perforant path fibers revealed direct, facilitating synaptic inputs from the entorhinal cortex. Taken together, these results indicate that NGFCs form an integral part of the local molecular layer microcircuitry generating feed-forward inhibition and provide a direct GABAergic pathway linking the dentate gyrus to the CA1 and subicular regions through the hippocampal fissure.
Copyright © 2010 Wiley-Liss, Inc.
Figures



References
-
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966;66:448–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

