Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons
- PMID: 21452215
- PMCID: PMC3233875
- DOI: 10.1002/cne.22576
Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons
Abstract
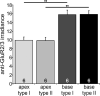
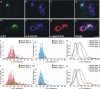
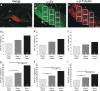
Inherent in the design of the mammalian auditory system is the precision necessary to transduce complex sounds and transmit the resulting electrical signals to higher neural centers. Unique specializations in the organ of Corti are required to make this conversion, such that mechanical and electrical properties of hair cell receptors are tailored to their specific role in signal coding. Electrophysiological and immunocytochemical characterizations have shown that this principle also applies to neurons of the spiral ganglion, as evidenced by distinctly different firing features and synaptic protein distributions of neurons that innervate high- and low-frequency regions of the cochlea. However, understanding the fine structure of how these properties are distributed along the cochlear partition and within the type I and type II classes of spiral ganglion neurons is necessary to appreciate their functional significance fully. To address this issue, we assessed the localization of the postsynaptic AMPA receptor subunits GluR2 and GluR3 and the presynaptic protein synaptophysin by using immunocytochemical labeling in both postnatal and adult tissue. We report that these presynaptic and postsynaptic proteins are distributed oppositely in relation to the tonotopic map and that they are equally distributed in each neuronal class, thus having an overall gradation from one end of the cochlea to the other. For synaptophysin, an additional layer of heterogeneity was superimposed orthogonal to the tonotopic axis. The highest anti-synaptophysin antibody levels were observed within neurons located close to the scala tympani compared with those located close to the scala vestibuli. Furthermore, we noted that the protein distribution patterns observed in postnatal preparations were largely retained in adult tissue sections, indicating that these features characterize spiral ganglion neurons in the fully developed ear.
Copyright © 2010 Wiley-Liss, Inc.
Figures










References
-
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002b;447:331–350. - PubMed
-
- Berglund AM, Benson TE, Brown MC. Synapses from labeled type II axons in the mouse cochlear nucleus. Hear Res. 1996;94:31–46. - PubMed
-
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

