Giant macrolactams based on β-sheet peptides
- PMID: 21452877
- PMCID: PMC3088102
- DOI: 10.1021/jo102598n
Giant macrolactams based on β-sheet peptides
Abstract
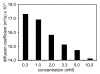
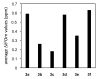
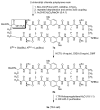
This paper reports the use of natural amino acids, the tripeptide β-strand mimic Hao, and the β-turn mimic δ-linked ornithine to generate water-soluble 54-, 78-, and 102-membered-ring macrolactams. These giant macrocycles were efficiently prepared by synthesis of the corresponding protected linear peptides, followed by solution-phase cyclization and deprotection. The protected linear peptide precursors were synthesized on 2-chlorotrityl chloride resin by conventional Fmoc-based solid-phase peptide synthesis. Macrocyclization was typically performed using HCTU and N,N-diisopropylethylamine in DMF at ca. 0.5 mM concentration. The macrocycles were isolated in 13-45% overall yield after HPLC purification and lyophilization. 1D, 2D TOCSY, and 2D ROESY (1)H NMR studies of the 54- and 78-membered-ring macrolactams establish that these compounds fold to form β-sheet structures in aqueous solutions.
© 2011 American Chemical Society
Figures


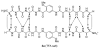


References
-
- Cascales L, Craik DJ. Org. Biomol. Chem. 2010;8:5035–5047. - PubMed
- Jagadish K, Camarero JA. Pept. Sci. 2010;94:611–616. - PMC - PubMed
- Gerlach SL, Rathinakumar R, Chakravarty G, Göransson U, Wimley WC, Darwin SP, Mondal D. Pept. Sci. 2010;94:617–625. - PubMed
- Mylne JS, Wang CK, van der Weerden NL, Craik DJ. Pept. Sci. 2010;94:635–646. - PubMed
- Colgrave ML, Korsinczky MJL, Clark RJ, Foley F, Craik DJ. Pept. Sci. 2010;94:665–672. - PubMed
-
- Kondejewski LH, Farmer SW, Wishart DS, Kay CM, Hancock REW, Hodges RS. J. Biol. Chem. 1996;271:25261–25268. - PubMed
- Gibbs AC, Kondejewski LH, Gronwald W, Nip AM, Hodges RS, Sykes BD, Wishart DS. Nat. Struct. Biol. 1998;5:284–288. - PubMed
- Gibbs AC, Bjorndahl TC, Hodges RS, Wishart DS. J. Am. Chem. Soc. 2002;124:1203–1213. - PubMed
- Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, Lu H, Yan X, Daly NL, Craik DJ, Jiang S, Lehrer RI, Blumenthal R. J. Biol. Chem. 2006;281:18787–18792. - PubMed
- Tran D, Tran P, Roberts K, Osapay G, Schaal J, Ouellette A, Selsted ME. Antimicrob. Agents Chemother. 2008;52:944–953. - PMC - PubMed
-
- Späth J, Stuart F, Jiang L, Robinson JA. Helv. Chim. Acta. 1998;81:1726–1738.
- Athanassiou Z, Patora K, Dias RLA, Moehle K, Robinson JA, Varani G. Biochemistry. 2006;46:741–751. - PubMed
- Fasan R, Dias RLA, Moehle K, Zerbe O, Obrecht D, Mittl PRE, Grütter MG, Robinson JA. ChemBioChem. 2006;7:515–526. - PubMed
- Robinson JA. Acc. Chem. Res. 2008;41:1278–1288. - PubMed
- Robinson JA, et al. Science. 2010;327:1010–1013.
-
- Conlan BF, Gillon AD, Craik DJ, Anderson MA. Pept. Sci. 2010;94:573–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

