Cerebral oxygenation in awake rats during acclimation and deacclimation to hypoxia: an in vivo electron paramagnetic resonance study
- PMID: 21452968
- PMCID: PMC3070067
- DOI: 10.1089/ham.2010.1038
Cerebral oxygenation in awake rats during acclimation and deacclimation to hypoxia: an in vivo electron paramagnetic resonance study
Abstract
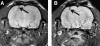
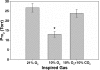
Exposure to high altitude or hypobaric hypoxia results in a series of metabolic, physiologic, and genetic changes that serve to acclimate the brain to hypoxia. Tissue Po(2) (Pto(2)) is a sensitive index of the balance between oxygen delivery and utilization and can be considered to represent the summation of such factors as cerebral blood flow, capillary density, hematocrit, arterial Po(2), and metabolic rate. As such, it can be used as a marker of the extent of acclimation. We developed a method using electron paramagnetic resonance (EPR) to measure Pto(2) in unanesthetized subjects with a chronically implanted sensor. EPR was used to measure rat cortical tissue Pto(2) in awake rats during acute hypoxia and over a time course of acclimation and deacclimation to hypobaric hypoxia. This was done to simulate the effects on brain Pto(2) of traveling to altitude for a limited period. Acute reduction of inspired O(2) to 10% caused a decline from 26.7 ± 2.2 to 13.0 ± 1.5 mmHg (mean ± SD). Addition of 10% CO(2) to animals breathing 10% O(2) returned Pto(2) to values measured while breathing 21% O(2,) indicating that hypercapnia can reverse the effects of acute hypoxia. Pto(2) in animals acclimated to 10% O(2) was similar to that measured preacclimation when breathing 21% O(2). Using a novel, individualized statistical model, it was shown that the T(1/2) of the Pto(2) response during exposure to chronic hypoxia was approximately 2 days. This indicates a capacity for rapid adaptation to hypoxia. When subjects were returned to normoxia, there was a transient hyperoxygenation, followed by a return to lower values with a T(1/2) of deacclimation of 1.5 to 3 days. These data indicate that exposure to hypoxia results in significant improvements in steady-state oxygenation for a given inspired O(2) and that both acclimation and deacclimation can occur within days.
Figures



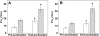
References
-
- Baumann R. Bauer C. Bartels H. Influence of chronic and acute hypoxia on oxygen affinity and red cell 2,3 diphosphoglycerate of rats and guinea pigs. Resp. Physiol. 1971;11:135–144. - PubMed
-
- Bereczki D. Wei L. Otsuka T. Hans F.J. Acuff V. Patlak C. Fenstermacher J. Hypercapnia slightly raises blood volume and sizably elevates flow velocity in brain microvessels. Am. J. Physiol. 1993;264:H1360–H1369. - PubMed
-
- Boero J.A. Ascher J. Arregui A. Rovainen C. Woolsey T.A. Increased brain capillaries in chronic hypoxia. J. Appl. Physiol. 1999;86:1211–1219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

