Gene therapy and targeted toxins for glioma
- PMID: 21453286
- PMCID: PMC3208509
- DOI: 10.2174/156652311795684722
Gene therapy and targeted toxins for glioma
Abstract
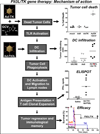
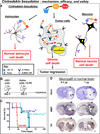
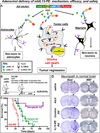
The most common primary brain tumor in adults is glioblastoma. These tumors are highly invasive and aggressive with a mean survival time of 15-18 months from diagnosis to death. Current treatment modalities are unable to significantly prolong survival in patients diagnosed with glioblastoma. As such, glioma is an attractive target for developing novel therapeutic approaches utilizing gene therapy. This review will examine the available preclinical models for glioma including xenographs, syngeneic and genetic models. Several promising therapeutic targets are currently being pursued in pre-clinical investigations. These targets will be reviewed by mechanism of action, i.e., conditional cytotoxic, targeted toxins, oncolytic viruses, tumor suppressors/oncogenes, and immune stimulatory approaches. Preclinical gene therapy paradigms aim to determine which strategies will provide rapid tumor regression and long-term protection from recurrence. While a wide range of potential targets are being investigated preclinically, only the most efficacious are further transitioned into clinical trial paradigms. Clinical trials reported to date are summarized including results from conditionally cytotoxic, targeted toxins, oncolytic viruses and oncogene targeting approaches. Clinical trial results have not been as robust as preclinical models predicted; this could be due to the limitations of the GBM models employed. Once this is addressed, and we develop effective gene therapies in models that better replicate the clinical scenario, gene therapy will provide a powerful approach to treat and manage brain tumors.
Figures

References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms-an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. - PubMed
-
- Stupp R, Hottinger AF, van den Bent MJ, Dietrich PY, Brandes AA. Frequently asked questions in the medical management of high-grade glioma: a short guide with practical answers. Ann Oncol. 2008;19 Suppl 7:vii209–vii216. - PubMed
-
- Stupp R, Roila F. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19 Suppl 2:ii83–ii85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054193/NS/NINDS NIH HHS/United States
- F32NS053034/NS/NINDS NIH HHS/United States
- 1F32 NS058156/NS/NINDS NIH HHS/United States
- 1R01-NS 054193/NS/NINDS NIH HHS/United States
- 1R01-NS061107/NS/NINDS NIH HHS/United States
- U01 NS052465/NS/NINDS NIH HHS/United States
- R01 NS061107/NS/NINDS NIH HHS/United States
- 1UO1 NS052465.01/NS/NINDS NIH HHS/United States
- 1R01-NS074387/NS/NINDS NIH HHS/United States
- R01 NS057711/NS/NINDS NIH HHS/United States
- R21 NS054143/NS/NINDS NIH HHS/United States
- 1R01-NS057711/NS/NINDS NIH HHS/United States
- R01 NS074387/NS/NINDS NIH HHS/United States
- 1R21-NS054143/NS/NINDS NIH HHS/United States
- F32 NS058156/NS/NINDS NIH HHS/United States
- F32 NS053034/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

