The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis
- PMID: 21453419
- PMCID: PMC3088984
- DOI: 10.1111/j.1365-2567.2011.03429.x
The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis
Abstract
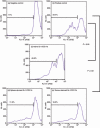
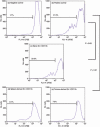
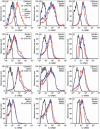
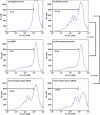
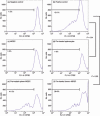
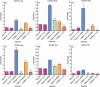
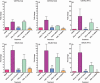
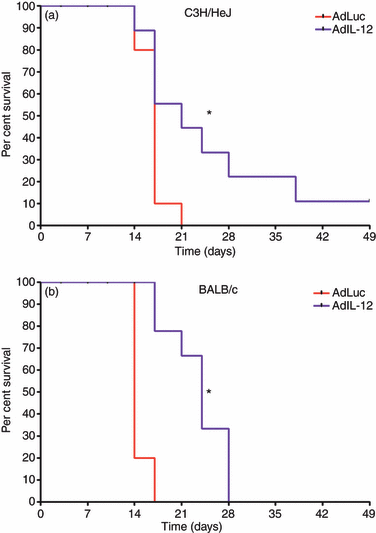
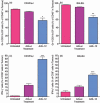
Myeloid-derived suppressor cells (MDSC) are important to the tumour microenvironment as they actively suppress the immune system and promote tumour progression and metastasis. These cells block T-cell activation in the tumour microenvironment, preventing anti-tumour immune activity. The ability of a treatment to alter the suppressive function of these cells and promote an immune response is essential to enhancing overall therapeutic efficacy. Interleukin-12 (IL-12) has the potential not only to promote anti-tumour immune responses but also to block the activity of cells capable of immune suppression. This paper identifies a novel role for IL-12 as a modulator of MDSC activity, with implications for IL-12 as a therapeutic agent. Treatment with IL-12 was found to alter the suppressive function of MDSC by fundamentally altering the cells. Interleukin-12-treated MDSC exhibited up-regulation of surface markers indicative of mature cells as well as decreases in nitric oxide synthase and interferon-γ mRNA both in vitro and in vivo. Treatment with IL-12 was also found to have significant therapeutic benefit by decreasing the percentage of MDSC in the tumour microenvironment and increasing the percentage of active CD8(+) T cells. Treatment with IL-12 resulted in an increase in overall survival accompanied by a reduction in metastasis. The findings in this paper identify IL-12 as a modulator of immune suppression with significant potential as a therapeutic agent for metastatic breast cancer.
© 2011 The Authors. Immunology © No claim to original US government works.
Figures
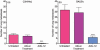
References
-
- Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. - PubMed
-
- Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte–macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

