The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence
- PMID: 21453433
- PMCID: PMC6640450
- DOI: 10.1111/j.1364-3703.2010.00681.x
The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence
Abstract
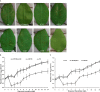
Xanthomonas citri ssp. citri (Xcc) causes citrus canker, one of the most economically damaging diseases affecting citrus worldwide. Biofilm formation is important for the pathogen to survive epiphytically in planta prior to the induction of canker symptoms. In this study, two EZ-Tn5 transposon mutants of Xcc strain 306, affected in biofilm formation, were isolated; subsequent analyses led to the identification of a novel gene locus XAC3596 (designated as wxacO), encoding a putative transmembrane protein, and the rfbC gene, encoding a truncated O-antigen biosynthesis protein. Sodium dodecylsulphate-polyacrylamide gel electrophoresis revealed that lipopolysaccharide (LPS) biosynthesis was affected in both wxacO and rfbC mutants. The wxacO mutant was impaired in the formation of a structured biofilm on glass or host plant leaves, as shown in confocal laser scanning microscopy analysis of strains containing a plasmid expressing the green fluorescent protein. Both wxacO and rfbC mutants were more sensitive than the wild-type strain to different environmental stresses, and more susceptible to the antimicrobial peptide polymyxin B. The two mutants were attenuated in swimming motility, but not in flagellar formation. The mutants also showed reduced virulence and decreased growth on host leaves when spray inoculated. The affected phenotypes of the wxacO and rfbC mutants were complemented to wild-type levels by the intact wxacO and rfbC genes, respectively. This report identifies a new gene influencing LPS production by Xcc. In addition, our results suggest that a structurally intact LPS is critical for survival in the phyllosphere and for the virulence of Xcc.
© 2010 THE AUTHORS. MOLECULAR PLANT PATHOLOGY © 2010 BSPP AND BLACKWELL PUBLISHING LTD.
Figures




References
-
- Berry, M.C. , McGhee, G.C. , Zhao, Y. and Sundin, G.W. (2009) Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora . FEMS Microbiol. Lett. 291, 80–87. - PubMed
-
- Boyer, H. and Roulland‐Dussoix, D. (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli . J. Mol. Biol. 41, 459–472. - PubMed
-
- Braun, S.G. , Meyer, A. , Holst, O. , Pühler, A. and Niehaus, K. (2005) Characterization of the Xanthomonas campestris pv. campestris lipopolysaccharide substructures essential for elicitation of an oxidative burst in tobacco cells. Mol. Plant–Microbe Interact. 18, 674–681. - PubMed
-
- Breton, C. , Snajdrova, L. , Jeanneau, C. , Koca, J. and Imberty, A. (2006) Structures and mechanisms of glycosyltransferases. Glycobiology, 16, 29R–37R. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

