Adjuvant therapy for locally advanced renal cell cancer: a systematic review with meta-analysis
- PMID: 21453469
- PMCID: PMC3080342
- DOI: 10.1186/1471-2407-11-115
Adjuvant therapy for locally advanced renal cell cancer: a systematic review with meta-analysis
Abstract
Background: Many adjuvant trials have been undertaken in an attempt to reduce the risk of recurrence among patients who undergo surgical resection for locally advanced renal cancer. However, no clear benefit has been identified to date. This systematic review was conducted to examine the exact role of adjuvant therapy in renal cancer setting.
Methods: Randomized controlled trials were searched comparing adjuvant therapy (chemotherapy, vaccine, immunotherapy, biochemotherapy) versus no active treatment after surgery among renal cell cancer patients. Outcomes were overall survival (OS), disease-free survival (DFS), and severe toxicities. Risk ratios (RR), hazard ratios (HR) and 95% confidence intervals were calculated using a fixed-effects meta-analysis. Heterogeneity was measured by I2. Different strategies of adjuvant treatment were evaluated separately.
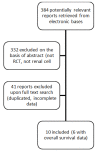
Results: Ten studies (2,609 patients) were included. Adjuvant therapy provided no benefits in terms of OS (HR 1.07; 95%CI 0.89 to 1.28; P = 0.48 I2 = 0%) or DFS (HR 1.03; 95%CI 0.87 to 1.21; P = 0.77 I2 = 15%) when compared to no treatment. No subgroup analysis (immunotherapy, vaccines, biochemotherapy and hormone therapy) had relevant results. Toxicity evaluation depicted a significantly higher frequency of serious adverse events in the adjuvant group.
Conclusions: This analysis provided no support for the hypothesis that the agents studied provide any clinical benefit for renal cancer patients although they increase the risk of toxic effects. Randomized trials are underway to test targeted therapies, which might open a new therapeutic frontier. Until these trials yield results, no adjuvant therapy can be recommended for patients who undergo surgical resection for renal cell cancer.
Figures
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Golimbu M, Joshi P, Sperber A, Tessler A, Al-Askari S, Morales P. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291–301. - PubMed
-
- Minasian LM, Motzer RJ, Gluck L, Mazumdar M, Vlamis V, Krown SE. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993;11:1368–1375. - PubMed
-
- Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–57. - PubMed
-
- Pizzocaro G, Piva L, Di Fronzo G, Giongo A, Cozzoli A, Dormia E, Minervini S, Zanollo A, Fontanella U, Longo G. et al. Adjuvant medroxyprogesterone acetate to radical nephrectomy in renal cancer: 5-year results of a prospective randomized study. J Urol. 1987;138:1379–1381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical