Insertion of the human sodium iodide symporter to facilitate deep tissue imaging does not alter oncolytic or replication capability of a novel vaccinia virus
- PMID: 21453532
- PMCID: PMC3080806
- DOI: 10.1186/1479-5876-9-36
Insertion of the human sodium iodide symporter to facilitate deep tissue imaging does not alter oncolytic or replication capability of a novel vaccinia virus
Abstract
Introduction: Oncolytic viruses show promise for treating cancer. However, to assess therapeutic efficacy and potential toxicity, a noninvasive imaging modality is needed. This study aimed to determine if insertion of the human sodium iodide symporter (hNIS) cDNA as a marker for non-invasive imaging of virotherapy alters the replication and oncolytic capability of a novel vaccinia virus, GLV-1h153.
Methods: GLV-1h153 was modified from parental vaccinia virus GLV-1h68 to carry hNIS via homologous recombination. GLV-1h153 was tested against human pancreatic cancer cell line PANC-1 for replication via viral plaque assays and flow cytometry. Expression and transportation of hNIS in infected cells was evaluated using Westernblot and immunofluorescence. Intracellular uptake of radioiodide was assessed using radiouptake assays. Viral cytotoxicity and tumor regression of treated PANC-1tumor xenografts in nude mice was also determined. Finally, tumor radiouptake in xenografts was assessed via positron emission tomography (PET) utilizing carrier-free 124I radiotracer.
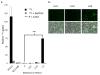
Results: GLV-1h153 infected, replicated within, and killed PANC-1 cells as efficiently as GLV-1h68. GLV-1h153 provided dose-dependent levels of hNIS expression in infected cells. Immunofluorescence detected transport of the protein to the cell membrane prior to cell lysis, enhancing hNIS-specific radiouptake (P < 0.001). In vivo, GLV-1h153 was as safe and effective as GLV-1h68 in regressing pancreatic cancer xenografts (P < 0.001). Finally, intratumoral injection of GLV-1h153 facilitated imaging of virus replication in tumors via 124I-PET.
Conclusion: Insertion of the hNIS gene does not hinder replication or oncolytic capability of GLV-1h153, rendering this novel virus a promising new candidate for the noninvasive imaging and tracking of oncolytic viral therapy.
Figures




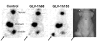
References
-
- Moss B. Poxviridae: the Viruses and Their Replication. 4. Philadelphia: Lippincott Williams & Wilkins; 2001.
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: WHO; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

