Cost-effectiveness of different human papillomavirus vaccines in Singapore
- PMID: 21453537
- PMCID: PMC3082243
- DOI: 10.1186/1471-2458-11-203
Cost-effectiveness of different human papillomavirus vaccines in Singapore
Abstract
Background: Human papillomavirus (HPV) vaccines are widely available and there have been studies exploring their potential clinical impact and cost-effectiveness. However, few studies have compared the cost-effectiveness among the 2 main vaccines available - a bivalent vaccine against HPV 16/18, and a quadrivalent vaccine against 6/11/16/18. We explore the cost-effectiveness of these two HPV vaccines in tropical Singapore.
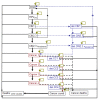
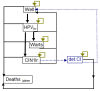
Methods: We developed a Markov state-transition model to represent the natural history of cervical cancer to predict HPV infection, cancer incidence, mortality, and costs. Cytologic screening and treatment of different outcomes of HPV infection were incorporated. Vaccination was provided to a cohort of 12-year old females in Singapore, followed up until death. Based on available vaccines on the market, the bivalent vaccine had increased effectiveness against a wider range of HPV types, while the quadrivalent vaccine had effectiveness against genital warts. Incremental cost-effectiveness ratios (ICER) compared vaccination to no-vaccination, and between the two vaccines. Sensitivity analyses explored differences in vaccine effectiveness and uptake, and other key input parameters.
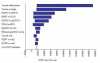
Results: For the no vaccination scenario, 229 cervical cancer cases occurred over the cohort's lifetime. The total discounted cost per individual due to HPV infection was SGD$275 with 28.54 discounted life-years. With 100% vaccine coverage, the quadrivalent vaccine reduced cancers by 176, and had an ICER of SGD$12,866 per life-year saved. For the bivalent vaccine, 197 cancers were prevented with an ICER of $12,827 per life-year saved. Comparing the bivalent to the quadrivalent vaccine, the ICER was $12,488 per life-year saved. However, the cost per QALY saved for the quadrivalent vaccine compared to no vaccine was $9,071, while it was $10,392 for the bivalent vaccine, with the quadrivalent vaccine dominating the bivalent vaccine due to the additional QALY effect from reduction in genital warts. The overall outcomes were most sensitive to vaccine cost and coverage.
Conclusion: HPV vaccination is a cost-effective strategy, and should be considered a possible strategy to reduce the impact of HPV infection.
Figures
References
-
- World Health Organization (WHO) Projections of mortality and burden of disease. http://www.who.int/healthinfo/global_burden_disease/projections/en/index... [Accessed 29th July 2009]
-
- Health Promotion Board. Singapore. Health Programmes - Cervical Screen Singapore. http://www.hpb.gov.sg/hpb/default.asp?pg_id=1741 [Accessed 27th July 2009]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical