A comparison of two worlds: How does Bayes hold up to the status quo for the analysis of clinical trials?
- PMID: 21453792
- PMCID: PMC4477745
- DOI: 10.1016/j.cct.2011.03.010
A comparison of two worlds: How does Bayes hold up to the status quo for the analysis of clinical trials?
Abstract
Background: There is a paucity of literature comparing Bayesian analytic techniques with traditional approaches for analyzing clinical trials using real trial data.
Methods: We compared Bayesian and frequentist group sequential methods using data from two published clinical trials. We chose two widely accepted frequentist rules, O'Brien-Fleming and Lan-DeMets, and conjugate Bayesian priors. Using the nonparametric bootstrap, we estimated a sampling distribution of stopping times for each method. Because current practice dictates the preservation of an experiment-wise false positive rate (Type I error), we approximated these error rates for our Bayesian and frequentist analyses with the posterior probability of detecting an effect in a simulated null sample. Thus for the data-generated distribution represented by these trials, we were able to compare the relative performance of these techniques.
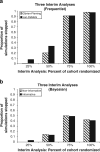
Results: No final outcomes differed from those of the original trials. However, the timing of trial termination differed substantially by method and varied by trial. For one trial, group sequential designs of either type dictated early stopping of the study. In the other, stopping times were dependent upon the choice of spending function and prior distribution.
Conclusions: Results indicate that trialists ought to consider Bayesian methods in addition to traditional approaches for analysis of clinical trials. Though findings from this small sample did not demonstrate either method to consistently outperform the other, they did suggest the need to replicate these comparisons using data from varied clinical trials in order to determine the conditions under which the different methods would be most efficient.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Friedman LM, Furberg CD, Demets DL. Fundamentals of Clinical Trials. Springer; New York: 1998.
-
- Hulley SB, Cummings SR, Browner WS, Grady D, Newman TB. Designing Clinical Research. Lippincott Williams & Wilkins; Wolters Kluer: 2006.
-
- Berry DA. Interim analyses in clinical trials: classical vs. Bayesian approaches. Stat Med. 1985;4:521–6. - PubMed
-
- Carlin BP, Louis TA. Bayesian Methods for Data Analysis. Chapman and Hall/CRC press; New York, New York: 2008.
-
- Freedman LS, Spiegelhalter DJ. Comparison of Bayesian with group sequential methods for monitoring clinical trials. Control Clin Trials. 1989;10:357–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

