Randomised prostate cancer screening trial: 20 year follow-up
- PMID: 21454449
- PMCID: PMC3069219
- DOI: 10.1136/bmj.d1539
Randomised prostate cancer screening trial: 20 year follow-up
Abstract
Objective: To assess whether screening for prostate cancer reduces prostate cancer specific mortality.
Design: Population based randomised controlled trial.
Setting: Department of Urology, Norrköping, and the South-East Region Prostate Cancer Register.
Participants: All men aged 50-69 in the city of Norrköping, Sweden, identified in 1987 in the National Population Register (n = 9026).
Intervention: From the study population, 1494 men were randomly allocated to be screened by including every sixth man from a list of dates of birth. These men were invited to be screened every third year from 1987 to 1996. On the first two occasions screening was done by digital rectal examination only. From 1993, this was combined with prostate specific antigen testing, with 4 µg/L as cut off. On the fourth occasion (1996), only men aged 69 or under at the time of the investigation were invited.
Main outcome measures: Data on tumour stage, grade, and treatment from the South East Region Prostate Cancer Register. Prostate cancer specific mortality up to 31 December 2008.
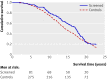
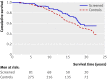
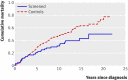
Results: In the four screenings from 1987 to 1996 attendance was 1161/1492 (78%), 957/1363 (70%), 895/1210 (74%), and 446/606 (74%), respectively. There were 85 cases (5.7%) of prostate cancer diagnosed in the screened group and 292 (3.9%) in the control group. The risk ratio for death from prostate cancer in the screening group was 1.16 (95% confidence interval 0.78 to 1.73). In a Cox proportional hazard analysis comparing prostate cancer specific survival in the control group with that in the screened group, the hazard ratio for death from prostate cancer was 1.23 (0.94 to 1.62; P = 0.13). After adjustment for age at start of the study, the hazard ratio was 1.58 (1.06 to 2.36; P = 0.024).
Conclusions: After 20 years of follow-up the rate of death from prostate cancer did not differ significantly between men in the screening group and those in the control group. Trial registration Current Controlled Trials, ISRCTN06342431.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
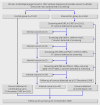
Comment in
-
Study raises five questions.BMJ. 2011 Jun 14;342:d3698. doi: 10.1136/bmj.d3698. BMJ. 2011. PMID: 21672978 No abstract available.
-
Evidence of overdiagnosis.BMJ. 2011 Jun 14;342:d3700. doi: 10.1136/bmj.d3700. BMJ. 2011. PMID: 21672979 No abstract available.
-
What is mortality denominator?BMJ. 2011 Jun 14;342:d3702. doi: 10.1136/bmj.d3702. BMJ. 2011. PMID: 21672980 No abstract available.
-
Report bias favours screening.BMJ. 2011 Jun 14;342:d3703. doi: 10.1136/bmj.d3703. BMJ. 2011. PMID: 21672981 No abstract available.
-
Study has major shortcomings.BMJ. 2011 Jun 14;342:d3704. doi: 10.1136/bmj.d3704. BMJ. 2011. PMID: 21672982 No abstract available.
-
Underscreened and undertreated.BMJ. 2011 Jun 14;342:d3708. doi: 10.1136/bmj.d3708. BMJ. 2011. PMID: 21672983 No abstract available.
-
Prostate cancer screening has no effect on prostate cancer specific mortality over 20 years of follow-up of Swedish men.Evid Based Med. 2012 Feb;17(1):25-6. doi: 10.1136/ebm-2011-100070. Epub 2011 Jul 6. Evid Based Med. 2012. PMID: 21733924 No abstract available.
-
Re: randomised prostate cancer screening trial: 20 year follow-up.Eur Urol. 2011 Dec;60(6):1306-7. doi: 10.1016/j.eururo.2011.08.070. Eur Urol. 2011. PMID: 22054407 No abstract available.
References
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al: ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
