Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States
- PMID: 21454450
- PMCID: PMC3112034
- DOI: 10.1182/blood-2010-11-316745
Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States
Abstract
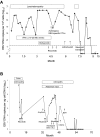
Chronic active EBV disease (CAEBV) is a lymphoproliferative disorder characterized by markedly elevated levels of antibody to EBV or EBV DNA in the blood and EBV RNA or protein in lymphocytes in tissues. We present our experience with CAEBV during the last 28 years, including the first 8 cases treated with hematopoietic stem cell transplantation in the United States. Most cases of CAEBV have been reported from Japan. Unlike CAEBV in Japan, where EBV is nearly always found in T or natural killer (NK) cells in tissues, EBV was usually detected in B cells in tissues from our patients. Most patients presented with lymphadenopathy and splenomegaly; fever, hepatitis, and pancytopenia were common. Most patients died of infection or progressive lymphoproliferation. Unlike cases reported from Japan, our patients often showed a progressive loss of B cells and hypogammaglobulinemia. Although patients with CAEBV from Japan have normal or increased numbers of NK cells, many of our patients had reduced NK-cell numbers. Although immunosuppressive agents, rituximab, autologous cytotoxic T cells, or cytotoxic chemotherapy often resulted in short-term remissions, they were not curative. Hematopoietic stem cell transplantation was often curative for CAEBV, even in patients with active lymphoproliferative disease that was unresponsive to chemotherapy. These studies are registered at http://www.clinicaltrials.gov as NCT00032513 for CAEBV, NCT00062868 and NCT00058812 for EBV-specific T-cell studies, and NCT00578539 for the hematopoietic stem cell transplantation protocol.
Figures



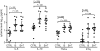
Similar articles
-
Spectrum of Epstein-Barr virus-associated T-cell lymphoproliferative disorder in adolescents and young adults in Taiwan.Int J Clin Exp Pathol. 2014 Apr 15;7(5):2430-7. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24966953 Free PMC article.
-
Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan.Am J Hematol. 2008 Sep;83(9):721-7. doi: 10.1002/ajh.21247. Am J Hematol. 2008. PMID: 18626884
-
Clinicopathological states of Epstein-Barr virus-associated T/NK-cell lymphoproliferative disorders (severe chronic active EBV infection) of children and young adults.Int J Oncol. 2004 May;24(5):1165-74. Int J Oncol. 2004. PMID: 15067338
-
Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency?Rev Med Virol. 2006 Jul-Aug;16(4):251-61. doi: 10.1002/rmv.505. Rev Med Virol. 2006. PMID: 16791843 Review.
-
Recent Concise Viewpoints of Chronic Active Epstein-Barr Virus Infection.Curr Pediatr Rev. 2015;11(1):5-9. doi: 10.2174/1573396311666150501002809. Curr Pediatr Rev. 2015. PMID: 25938379 Review.
Cited by
-
Sequential monitoring of serum IL-6, TNF-α, and IFN-γ levels in a CAEBV patient treated by plasma exchange and immunochemotherapy.Int J Hematol. 2012 Nov;96(5):669-73. doi: 10.1007/s12185-012-1170-2. Epub 2012 Sep 16. Int J Hematol. 2012. PMID: 22983646
-
Validation of Multiplex Serology detecting human herpesviruses 1-5.PLoS One. 2018 Dec 27;13(12):e0209379. doi: 10.1371/journal.pone.0209379. eCollection 2018. PLoS One. 2018. PMID: 30589867 Free PMC article.
-
Epstein-Barr virus infection: the micro and macro worlds.Virol J. 2023 Oct 2;20(1):220. doi: 10.1186/s12985-023-02187-9. Virol J. 2023. PMID: 37784180 Free PMC article. Review.
-
Dual Threat of Epstein-Barr Virus: an Autopsy Case Report of HIV-Positive Plasmablastic Lymphoma Complicating EBV-Associated Hemophagocytic Lymphohistiocytosis.J Clin Immunol. 2018 May;38(4):478-483. doi: 10.1007/s10875-018-0500-4. Epub 2018 Apr 23. J Clin Immunol. 2018. PMID: 29687211
-
T Cell-Epstein-Barr Virus-Associated Hemophagocytic Lymphohistiocytosis (HLH) Occurs in Non-Asians and Is Associated with a T Cell Activation State that Is Comparable to Primary HLH.J Clin Immunol. 2021 Oct;41(7):1582-1596. doi: 10.1007/s10875-021-01073-9. Epub 2021 Jun 26. J Clin Immunol. 2021. PMID: 34173902 Free PMC article.
References
-
- Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98(2):280–286. - PubMed
-
- Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80(1):64–69. - PubMed
-
- Kimura H, Morishima T, Kanegame H, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infec Dis. 2003;187(4):527–533. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

