Rap1-GTP-interacting adaptor molecule (RIAM) protein controls invasion and growth of melanoma cells
- PMID: 21454517
- PMCID: PMC3099666
- DOI: 10.1074/jbc.M110.189811
Rap1-GTP-interacting adaptor molecule (RIAM) protein controls invasion and growth of melanoma cells
Abstract
The Mig-10/RIAM/lamellipodin (MRL) family member Rap1-GTP-interacting adaptor molecule (RIAM) interacts with active Rap1, a small GTPase that is frequently activated in tumors such as melanoma and prostate cancer. We show here that RIAM is expressed in metastatic human melanoma cells and that both RIAM and Rap1 are required for BLM melanoma cell invasion. RIAM silencing in melanoma cells led to inhibition of tumor growth and to delayed metastasis in a severe combined immunodeficiency xenograft model. Defective invasion of RIAM-silenced melanoma cells arose from impairment in persistent cell migration directionality, which was associated with deficient activation of a Vav2-RhoA-ROCK-myosin light chain pathway. Expression of constitutively active Vav2 and RhoA in cells depleted for RIAM partially rescued their invasion, indicating that Vav2 and RhoA mediate RIAM function. These results suggest that inhibition of cell invasion in RIAM-silenced melanoma cells is likely based on altered cell contractility and cell polarization. Furthermore, we show that RIAM depletion reduces β1 integrin-dependent melanoma cell adhesion, which correlates with decreased activation of both Erk1/2 MAPK and phosphatidylinositol 3-kinase, two central molecules controlling cell growth and cell survival. In addition to causing inhibition of cell proliferation, RIAM silencing led to higher susceptibility to cell apoptosis. Together, these data suggest that defective activation of these kinases in RIAM-silenced cells could account for inhibition of melanoma cell growth and that RIAM might contribute to the dissemination of melanoma cells.
Figures
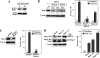


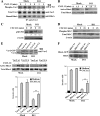




References
-
- Bos J. L. (2005) Curr. Opin. Cell Biol. 17, 123–128 - PubMed
-
- Bos J. L., Rehmann H., Wittinghofer A. (2007) Cell 129, 865–877 - PubMed
-
- Lafuente E. M., van Puijenbroek A. A., Krause M., Carman C. V., Freeman G. J., Berezovskaya A., Constantine E., Springer T. A., Gertler F. B., Boussiotis V. A. (2004) Dev. Cell 7, 585–595 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

