FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit
- PMID: 21454534
- PMCID: PMC3099672
- DOI: 10.1074/jbc.M110.184101
FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit
Abstract
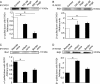
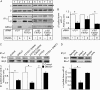
The seven members of the FXYD protein family associate with the Na(+)-K(+) pump and modulate its activity. We investigated whether conserved cysteines in FXYD proteins are susceptible to glutathionylation and whether such reactivity affects Na(+)-K(+) pump function in cardiac myocytes and Xenopus oocytes. Glutathionylation was detected by immunoblotting streptavidin precipitate from biotin-GSH loaded cells or by a GSH antibody. Incubation of myocytes with recombinant FXYD proteins resulted in competitive displacement of native FXYD1. Myocyte and Xenopus oocyte pump currents were measured with whole-cell and two-electrode voltage clamp techniques, respectively. Native FXYD1 in myocytes and FXYD1 expressed in oocytes were susceptible to glutathionylation. Mutagenesis identified the specific cysteine in the cytoplasmic terminal that was reactive. Its reactivity was dependent on flanking basic amino acids. We have reported that Na(+)-K(+) pump β(1) subunit glutathionylation induced by oxidative signals causes pump inhibition in a previous study. In the present study, we found that β(1) subunit glutathionylation and pump inhibition could be reversed by exposing myocytes to exogenous wild-type FXYD3. A cysteine-free FXYD3 derivative had no effect. Similar results were obtained with wild-type and mutant FXYD proteins expressed in oocytes. Glutathionylation of the β(1) subunit was increased in myocardium from FXYD1(-/-) mice. In conclusion, there is a dependence of Na(+)-K(+) pump regulation on reactivity of two specifically identified cysteines on separate components of the multimeric Na(+)-K(+) pump complex. By facilitating deglutathionylation of the β(1) subunit, FXYD proteins reverse oxidative inhibition of the Na(+)-K(+) pump and play a dynamic role in its regulation.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

