Phosphomimetic substitution of heterogeneous nuclear ribonucleoprotein A1 at serine 199 abolishes AKT-dependent internal ribosome entry site-transacting factor (ITAF) function via effects on strand annealing and results in mammalian target of rapamycin complex 1 (mTORC1) inhibitor sensitivity
- PMID: 21454539
- PMCID: PMC3091246
- DOI: 10.1074/jbc.M110.205096
Phosphomimetic substitution of heterogeneous nuclear ribonucleoprotein A1 at serine 199 abolishes AKT-dependent internal ribosome entry site-transacting factor (ITAF) function via effects on strand annealing and results in mammalian target of rapamycin complex 1 (mTORC1) inhibitor sensitivity
Abstract
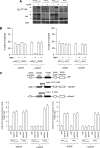
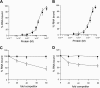
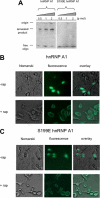
The relative activity of the AKT kinase has been demonstrated to be a major determinant of sensitivity of tumor cells to mammalian target of rapamycin (mTOR) complex 1 inhibitors. Our previous studies have shown that the multifunctional RNA-binding protein heterogeneous nuclear ribonucleoprotein (hnRNP) A1 regulates a salvage pathway facilitating internal ribosome entry site (IRES)-dependent mRNA translation of critical cellular determinants in an AKT-dependent manner following mTOR inhibitor exposure. This pathway functions by stimulating IRES-dependent translation in cells with relatively quiescent AKT, resulting in resistance to rapamycin. However, the pathway is repressed in cells with elevated AKT activity, rendering them sensitive to rapamycin-induced G(1) arrest as a result of the inhibition of global eIF-4E-mediated translation. AKT phosphorylation of hnRNP A1 at serine 199 has been demonstrated to inhibit IRES-mediated translation initiation. Here we describe a phosphomimetic mutant of hnRNP A1 (S199E) that is capable of binding both the cyclin D1 and c-MYC IRES RNAs in vitro but lacks nucleic acid annealing activity, resulting in inhibition of IRES function in dicistronic mRNA reporter assays. Utilizing cells in which AKT is conditionally active, we demonstrate that overexpression of this mutant renders quiescent AKT-containing cells sensitive to rapamycin in vitro and in xenografts. We also demonstrate that activated AKT is strongly correlated with elevated Ser(P)(199)-hnRNP A1 levels in a panel of 22 glioblastomas. These data demonstrate that the phosphorylation status of hnRNP A1 serine 199 regulates the AKT-dependent sensitivity of cells to rapamycin and functionally links IRES-transacting factor annealing activity to cellular responses to mTOR complex 1 inhibition.
Figures
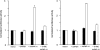

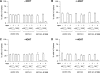
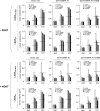

References
-
- Shi Y., Gera J., Hu L., Hsu J. H., Bookstein R., Li W., Lichtenstein A. (2002) Cancer Res. 62, 5027–5034 - PubMed
-
- Gera J. F., Mellinghoff I. K., Shi Y., Rettig M. B., Tran C., Hsu J. H., Sawyers C. L., Lichtenstein A. K. (2004) J. Biol. Chem. 279, 2737–2746 - PubMed
-
- Shi Y., Sharma A., Wu H., Lichtenstein A., Gera J. (2005) J. Biol. Chem. 280, 10964–10973 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

