Impairment of human immunodeficiency virus type-1 integrase SUMOylation correlates with an early replication defect
- PMID: 21454548
- PMCID: PMC3121452
- DOI: 10.1074/jbc.M110.189274
Impairment of human immunodeficiency virus type-1 integrase SUMOylation correlates with an early replication defect
Abstract
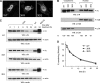
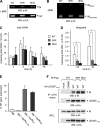
HIV-1 integrase (IN) orchestrates the integration of the reverse transcribed viral cDNA into the host cell genome and participates also in other steps of HIV-1 replication. Cellular and viral factors assist IN in performing its multiple functions, and post-translational modifications contribute to modulate its activities. Here, we show that HIV-1 IN is modified by SUMO proteins and that phylogenetically conserved SUMOylation consensus motifs represent major SUMO acceptor sites. Viruses harboring SUMOylation site IN mutants displayed a replication defect that was mapped during the early stages of infection, before integration but after reverse transcription. Because SUMOylation-defective IN mutants retained WT catalytic activity, we hypothesize that SUMOylation might regulate the affinity of IN for co-factors, contributing to efficient HIV-1 replication.
Figures


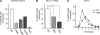
References
-
- Craigie R. (2001) J. Biol. Chem. 276, 23213–23216 - PubMed
-
- Esposito D., Craigie R. (1999) Adv. Virus. Res. 52, 319–333 - PubMed
-
- Vandegraaff N., Engelman A. (2007) Expert Rev. Mol. Med. 9, 1–19 - PubMed
-
- Craigie R., Fujiwara T., Bushman F. (1990) Cell 62, 829–837 - PubMed
-
- Katz R. A., Merkel G., Kulkosky J., Leis J., Skalka A. M. (1990) Cell 63, 87–95 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

