Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans
- PMID: 21454551
- PMCID: PMC3089554
- DOI: 10.1074/jbc.M110.208082
Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans
Abstract
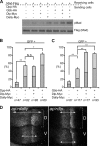
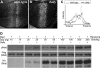
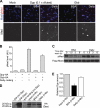
We previously proposed a model that DALLY, a Drosophila glypican, acts as a trans co-receptor to regulate BMP signaling in the germ line stem cell niche. To investigate the molecular mechanisms of contact-dependent BMP signaling, we developed novel in vitro assay systems to monitor trans signaling using Drosophila S2 cells. Using immunoblot-based as well as single-cell assay systems, we present evidence that Drosophila glypicans indeed enhance BMP signaling in trans in a contact-dependent manner in vitro. Our analysis showed that heparan sulfate modification is required for the trans co-receptor activity of DALLY. Two BMP-like molecules, Decapentaplegic (DPP) and Glass bottom boat, can mediate trans signaling through a heparan sulfate proteoglycan co-receptor in S2 cells. The in vitro systems reflect the molecular characteristics of heparan sulfate proteoglycan functions observed previously in vivo, such as ligand specificity and biphasic activity dependent on the ligand dosage. In addition, experiments using a DALLY-coated surface suggested that DALLY regulates DPP signaling in trans by its effect on the stability of DPP protein on the surface of the contacting cells. Our findings provide the molecular foundation for novel contact-dependent signaling, which defines the physical space of the stem cell niche in vivo.
Figures

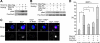
References
-
- Jackson S. M., Nakato H., Sugiura M., Jannuzi A., Oakes R., Kaluza V., Golden C., Selleck S. B. (1997) Development 124, 4113–4120 - PubMed
-
- Fujise M., Takeo S., Kamimura K., Matsuo T., Aigaki T., Izumi S., Nakato H. (2003) Development 130, 1515–1522 - PubMed
-
- Belenkaya T. Y., Han C., Yan D., Opoka R. J., Khodoun M., Liu H., Lin X. (2004) Cell 119, 231–244 - PubMed
-
- Kirkpatrick C. A., Selleck S. B. (2007) J. Cell Sci. 120, 1829–1832 - PubMed
-
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. (1991) Cell 64, 841–848 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

