Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2
- PMID: 21454556
- PMCID: PMC3089563
- DOI: 10.1074/jbc.M111.229419
Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2
Abstract
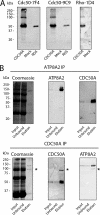
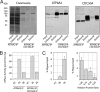
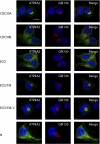
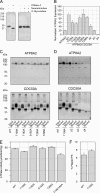
P(4)-ATPases have been implicated in the transport of lipids across cellular membranes. Some P(4)-ATPases are known to associate with members of the CDC50 protein family. Previously, we have shown that the P(4)-ATPase ATP8A2 purified from photoreceptor membranes and reconstituted into liposomes catalyzes the active transport of phosphatidylserine across membranes. However, it was unclear whether ATP8A2 functioned alone or as a complex with a CDC50 protein. Here, we show by mass spectrometry and Western blotting using newly generated anti-CDC50A antibodies that CDC50A is associated with ATP8A2 purified from photoreceptor membranes. ATP8A2 expressed in HEK293T cells assembles with endogenous or expressed CDC50A, but not CDC50B, to generate a heteromeric complex that actively transports phosphatidylserine and to a lesser extent phosphatidylethanolamine across membranes. Chimera CDC50 proteins in which various domains of CDC50B were replaced with the corresponding domains of CDC50A were used to identify domains important in the formation of a functional ATP8A2-CDC50 complex. These studies indicate that both the transmembrane and exocytoplasmic domains of CDC50A are required to generate a functionally active complex. The N-terminal cytoplasmic domain of CDC50A appears to play a direct role in the reaction cycle. Mutagenesis studies further indicate that the N-linked oligosaccharide chains of CDC50A are required for stable expression of an active ATP8A2-CDC50A lipid transport complex. Together, our studies indicate that CDC50A is the β-subunit of ATP8A2 and is crucial for the correct folding, stable expression, export from endoplasmic reticulum, and phosphatidylserine flippase activity of ATP8A2.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

