Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance
- PMID: 21454566
- PMCID: PMC3093820
- DOI: 10.1074/jbc.M110.215434
Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance
Abstract
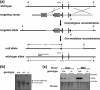
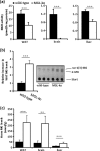
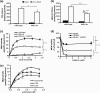
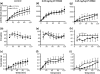
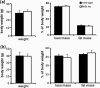
Monoglyceride lipase (MGL) influences energy metabolism by at least two mechanisms. First, it hydrolyzes monoacylglycerols (MG) into fatty acids and glycerol. These products can be used for energy production or synthetic reactions. Second, MGL degrades 2-arachidonoyl glycerol (2-AG), the most abundant endogenous ligand of cannabinoid receptors (CBR). Activation of CBR affects energy homeostasis by central orexigenic stimuli, by promoting lipid storage, and by reducing energy expenditure. To characterize the metabolic role of MGL in vivo, we generated an MGL-deficient mouse model (MGL-ko). These mice exhibit a reduction in MG hydrolase activity and a concomitant increase in MG levels in adipose tissue, brain, and liver. In adipose tissue, the lack of MGL activity is partially compensated by hormone-sensitive lipase. Nonetheless, fasted MGL-ko mice exhibit reduced plasma glycerol and triacylglycerol, as well as liver triacylglycerol levels indicative for impaired lipolysis. Despite a strong elevation of 2-AG levels, MGL-ko mice exhibit normal food intake, fat mass, and energy expenditure. Yet mice lacking MGL show a pharmacological tolerance to the CBR agonist CP 55,940 suggesting that the elevated 2-AG levels are functionally antagonized by desensitization of CBR. Interestingly, however, MGL-ko mice receiving a high fat diet exhibit significantly improved glucose tolerance and insulin sensitivity in comparison with wild-type controls despite equal weight gain. In conclusion, our observations implicate that MGL deficiency impairs lipolysis and attenuates diet-induced insulin resistance. Defective degradation of 2-AG does not provoke cannabinoid-like effects on feeding behavior, lipid storage, and energy expenditure, which may be explained by desensitization of CBR.
© 2011 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

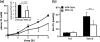
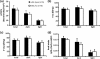

References
-
- Lowe M. E. (1997) Annu. Rev. Nutr. 17, 141–158 - PubMed
-
- Goldberg I. J., Merkel M. (2001) Front. Biosci. 6, D388–405 - PubMed
-
- Gao Y., Vasilyev D. V., Goncalves M. B., Howell F. V., Hobbs C., Reisenberg M., Shen R., Zhang M. Y., Strassle B. W., Lu P., Mark L., Piesla M. J., Deng K., Kouranova E. V., Ring R. H., Whiteside G. T., Bates B., Walsh F. S., Williams G., Pangalos M. N., Samad T. A., Doherty P. (2010) J. Neurosci. 30, 2017–2024 - PMC - PubMed
-
- Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Science 306, 1383–1386 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

