Recruitment of class I hydrophobins to the air:water interface initiates a multi-step process of functional amyloid formation
- PMID: 21454575
- PMCID: PMC3091204
- DOI: 10.1074/jbc.M110.214197
Recruitment of class I hydrophobins to the air:water interface initiates a multi-step process of functional amyloid formation
Abstract
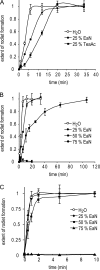
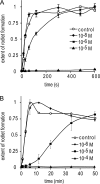
Class I fungal hydrophobins form amphipathic monolayers composed of amyloid rodlets. This is a remarkable case of functional amyloid formation in that a hydrophobic:hydrophilic interface is required to trigger the self-assembly of the proteins. The mechanism of rodlet formation and the role of the interface in this process have not been well understood. Here, we have studied the effect of a range of additives, including ionic liquids, alcohols, and detergents, on rodlet formation by two class I hydrophobins, EAS and DewA. Although the conformation of the hydrophobins in these different solutions is not altered, we observe that the rate of rodlet formation is slowed as the surface tension of the solution is decreased, regardless of the nature of the additive. These results suggest that interface properties are of critical importance for the recruitment, alignment, and structural rearrangement of the amphipathic hydrophobin monomers. This work gives insight into the forces that drive macromolecular assembly of this unique family of proteins and allows us to propose a three-stage model for the interface-driven formation of rodlets.
Figures




References
-
- Linder M. B., Szilvay G. R., Nakari-Setälä T., Penttilä M. E. (2005) FEMS Microbiol. Rev. 29, 877–896 - PubMed
-
- Sunde M., Kwan A. H., Templeton M. D., Beever R. E., Mackay J. P. (2008) Micron 39, 773–784 - PubMed
-
- Wösten H. A. (2001) Annu. Rev. Microbiol. 55, 625–646 - PubMed
-
- Talbot N. J. (2003) Current Biology 13, R696-R698 - PubMed
-
- Talbot N. J. (1997) Current Biology 7, R78-R81 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

