DiGeorge critical region 8 (DGCR8) is a double-cysteine-ligated heme protein
- PMID: 21454614
- PMCID: PMC3089513
- DOI: 10.1074/jbc.M110.180844
DiGeorge critical region 8 (DGCR8) is a double-cysteine-ligated heme protein
Abstract
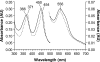
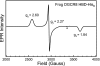
All known heme-thiolate proteins ligate the heme iron using one cysteine side chain. We previously found that DiGeorge Critical Region 8 (DGCR8), an essential microRNA processing factor, associates with heme of unknown redox state when overexpressed in Escherichia coli. On the basis of the similarity of the 450-nm Soret absorption peak of the DGCR8-heme complex to that of cytochrome P450 containing ferrous heme with CO bound, we identified cysteine 352 as a probable axial ligand in DGCR8. Here we further characterize the DGCR8-heme interaction using biochemical and spectroscopic methods. The DGCR8-heme complex is highly stable, with a half-life exceeding 4 days. Mutation of the conserved proline 351 to an alanine increases the rate of heme dissociation and allows the DGCR8-heme complex to be reconstituted biochemically. Surprisingly, DGCR8 binds ferric heme without CO to generate a hyperporphyrin spectrum. The electronic absorption, magnetic circular dichroism, and electron paramagnetic resonance spectra of the DGCR8-heme complex suggest a ferric heme bearing two cysteine ligands. This model was further confirmed using selenomethionine-substituted DGCR8 and mercury titration. DGCR8 is the first example of a heme-binding protein with two endogenous cysteine side chains serving as axial ligands. We further show that native DGCR8 binds heme when expressed in eukaryotic cells. This study provides a chemical basis for understanding the function of the DGCR8-heme interaction in microRNA maturation.
Figures








References
-
- Perutz M. F., Wilkinson A. J., Paoli M., Dodson G. G. (1998) Annu. Rev. Biophys. Biomol. Struct. 27, 1–34 - PubMed
-
- Derbyshire E. R., Marletta M. A. (2009) Handb. Exp. Pharmacol. 191, 17–31 - PubMed
-
- Ortiz de Montellano P. R. (2000) Curr. Opin. Chem. Biol. 4, 221–227 - PubMed
-
- Ortiz de Montellano P. R. (2004) Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd Ed., Springer, New York
-
- Dawson J. H., Sono M. (1987) Chem. Rev. 87, 1255–1276
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

