Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection
- PMID: 21454634
- PMCID: PMC3099705
- DOI: 10.1074/jbc.M110.206110
Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection
Abstract
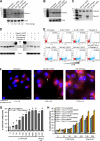
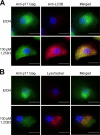
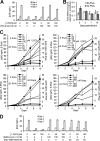
Autophagy is a self-digestion pathway essential for maintaining cellular homeostasis and cell survival and for degrading intracellular pathogens. Human immunodeficiency virus-1 (HIV-1) may utilize autophagy for replication as the autophagy-related protein-7 (ATG-7), microtubule-associated protein 1 light chain 3, ATG-12, and ATG-16L2 are required for productive HIV-1 infection; however, the effects of autophagy induction on HIV-1 infection are unknown. HIV-1-infected individuals have lower levels of 1α,25-dihydroxycholecalciferol, the hormonally active form of vitamin D, than uninfected individuals. with the lowest concentrations found in persons with AIDS. Using human macrophages and RNA interference for ATG-5 and Beclin-1 and chemical inhibition of phosphatidylinositol 3-kinase, we have found that physiologically relevant concentrations of 1α,25-dihydroxycholecalciferol induce autophagy in human macrophages through a phosphatidylinositol 3-kinase-, ATG-5-, and Beclin-1-dependent mechanism that significantly inhibits HIV-1 replication in a dose-dependent manner. We also show that the inhibition of basal autophagy inhibits HIV-1 replication. Furthermore, although 1α,25-dihydroxycholecalciferol induces the secretion of human cathelicidin, at the concentrations produced in vitro, cathelicidin does not trigger autophagy. Our findings support an important role for autophagy during HIV-1 infection and provide new insights into novel approaches to prevent and treat HIV-1 infection and related opportunistic infections.
Figures





References
-
- Joint United Nations Programme on HIV/AIDS (2008) Report on the Global HIV/AIDS Epidemic 2008, Executive Summary, Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland
-
- Martineau A. R., Honecker F. U., Wilkinson R. J., Griffiths C. J. (2007) J. Steroid Biochem. Mol. Biol. 103, 793–798 - PubMed
-
- Sly L. M., Lopez M., Nauseef W. M., Reiner N. E. (2001) J. Biol. Chem. 276, 35482–35493 - PubMed
-
- Yuk J. M., Shin D. M., Lee H. M., Yang C. S., Jin H. S., Kim K. K., Lee Z. W., Lee S. H., Kim J. M., Jo E. K. (2009) Cell Host. Microbe 6, 231–243 - PubMed
-
- Haug C., Müller F., Aukrust P., Frøland S. S. (1994) J. Infect. Dis. 169, 889–893 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

