Direct evidence for methyl group coordination by carbon-oxygen hydrogen bonds in the lysine methyltransferase SET7/9
- PMID: 21454678
- PMCID: PMC3099682
- DOI: 10.1074/jbc.M111.232876
Direct evidence for methyl group coordination by carbon-oxygen hydrogen bonds in the lysine methyltransferase SET7/9
Abstract
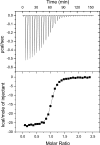
SET domain lysine methyltransferases (KMTs) are S-adenosylmethionine (AdoMet)-dependent enzymes that catalyze the site-specific methylation of lysyl residues in histone and non-histone proteins. Based on crystallographic and cofactor binding studies, carbon-oxygen (CH · · · O) hydrogen bonds have been proposed to coordinate the methyl groups of AdoMet and methyllysine within the SET domain active site. However, the presence of these hydrogen bonds has only been inferred due to the uncertainty of hydrogen atom positions in x-ray crystal structures. To experimentally resolve the positions of the methyl hydrogen atoms, we used NMR (1)H chemical shift coupled with quantum mechanics calculations to examine the interactions of the AdoMet methyl group in the active site of the human KMT SET7/9. Our results indicated that at least two of the three hydrogens in the AdoMet methyl group engage in CH · · · O hydrogen bonding. These findings represent direct, quantitative evidence of CH · · · O hydrogen bond formation in the SET domain active site and suggest a role for these interactions in catalysis. Furthermore, thermodynamic analysis of AdoMet binding indicated that these interactions are important for cofactor binding across SET domain enzymes.
Figures

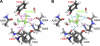

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

