Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy
- PMID: 21454723
- PMCID: PMC3087768
- DOI: 10.2215/CJN.09291010
Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy
Abstract
Background and objectives: In ADOPT (A Diabetes Outcomes Prevention Trial), initial monotherapy with rosiglitazone provided more durable glycemic control than metformin or glyburide in patients with recently diagnosed type 2 diabetes. Herein, we examine differences in albumin excretion, renal function (estimated GFR), and BP over 5 years between treatment groups.
Design, setting, participants, & measurements: A total of 4351 recently diagnosed, drug-naïve patients with type 2 diabetes were treated and followed for up to 5 years with rosiglitazone, metformin, or glyburide and were examined with periodic assessments of albumin/creatinine ratio (ACR), modification of diet in renal disease (MDRD)-estimated GFR, and BP.
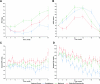
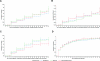
Results: The ACR rose slowly with metformin. It fell with rosiglitazone and less so with glyburide over the first 2 years, and then rose slowly over time. Estimated GFR (eGFR) with all therapies rose into the high normal range over the first 3 to 4 years, more so with rosiglitazone, and then declined, more so with glyburide. Systolic BP was stable over time, values with rosiglitazone being lower, and diastolic BP declined over time, more so with rosiglitazone than with metformin or glyburide. There was no difference among groups in the incidence of emergent albuminuria (ACR ≥30 mg/g), hypertension, or impaired eGFR (<60 ml/min per 1.73 m(2)).
Conclusions: Over a 5-year period, initial monotherapy with rosiglitazone retards the rise of ACR compared with metformin, preserves eGFR compared with glyburide, and lowers BP relative to both comparators.
Trial registration: ClinicalTrials.gov NCT00279045.
Copyright © 2011 by the American Society of Nephrology
Figures
References
-
- Karalliedde J, Viberti G: Microalbuminuria and cardiovascular risk. Am J Hypertens 17: 986–993, 2004 - PubMed
-
- Diabetes Control and Complications Trial (DCCT) Research Group: Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. The Diabetes Control and Complications (DCCT) Research Group. Kidney Int 47: 1703–1720 1995 - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577–1589, 2008 - PubMed
-
- Wang PH, Lau J, Chalmers TC: Meta-analysis of effects of intensive blood glucose control on late complications of type 1 diabetes. Lancet 341: 1306–1309, 1993 - PubMed
-
- Guan Y, Breyer MD: Peroxisome proliferator-activated receptors (PPARs): Novel therapeutic targets in renal disease. Kidney Int 60: 14–30, 2001 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

