Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation
- PMID: 21454805
- PMCID: PMC3262680
- DOI: 10.1165/rcmb.2010-0113OC
Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation
Abstract
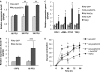
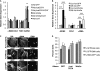
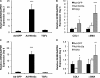
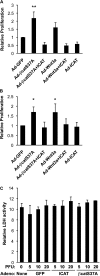
Pulmonary fibrosis is a disease that results in loss of normal lung architecture, but the signaling events that drive tissue destruction are incompletely understood. Wnt/β-catenin signaling is important in normal lung development, but whether abnormal signaling occurs in lung fibrosis due to systemic sclerosis and the consequences of β-catenin signaling toward the fibrogenic phenotype remain poorly defined. In this study, we show nuclear β-catenin accumulation in fibroblastic foci from lungs of patients with systemic sclerosis-associated advanced pulmonary fibrosis. Forced activation of β-catenin signaling in three independently derived sources of normal human lung fibroblasts promotes proliferation and migratory activities but is not sufficient to activate classic markers of fibroblast activation, such as TGF-β, type 1 collagen, α-smooth muscle actin, and connective tissue growth factor. These findings indicate that activation of β-catenin signaling in pulmonary fibroblasts may be a common feature of lung fibrosis, contributing to fibroproliferative and migratory activities associated with the disease.
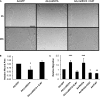
Figures

References
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151 - PubMed
-
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest 2002; 122(Suppl)286S–289S - PubMed
-
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004;5:691–701 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES013995/ES/NIEHS NIH HHS/United States
- F32 HL078145/HL/NHLBI NIH HHS/United States
- HL094643/HL/NHLBI NIH HHS/United States
- R01 AR042309/AR/NIAMS NIH HHS/United States
- HL071643/HL/NHLBI NIH HHS/United States
- R01 HL094643/HL/NHLBI NIH HHS/United States
- R01 ES015024/ES/NIEHS NIH HHS/United States
- P30 HL101292/HL/NHLBI NIH HHS/United States
- GM076561/GM/NIGMS NIH HHS/United States
- AR050840/AR/NIAMS NIH HHS/United States
- K08 HL093216/HL/NHLBI NIH HHS/United States
- ES015024/ES/NIEHS NIH HHS/United States
- AR42309/AR/NIAMS NIH HHS/United States
- ES013995/ES/NIEHS NIH HHS/United States
- HL78145/HL/NHLBI NIH HHS/United States
- R01 AR050840/AR/NIAMS NIH HHS/United States
- R01 GM076561/GM/NIGMS NIH HHS/United States
- R56 AR042309/AR/NIAMS NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 AR049025/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

