Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages
- PMID: 21454810
- PMCID: PMC3098301
- DOI: 10.1161/ATVBAHA.110.222547
Runx2-upregulated receptor activator of nuclear factor κB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages
Abstract
Objective: Clinical and experimental studies demonstrate the important roles of vascular smooth muscle cells (VSMC) in the pathogenesis of atherosclerosis. We have previously determined that the osteogenic transcription factor Runx2 is essential for VSMC calcification. The present study characterized Runx2-regulated signals and their potential roles in vascular calcification.
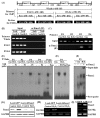
Methods and results: In vivo studies with atherogenic apolipoprotein E(-/-) mice demonstrated that increased oxidative stress was associated with upregulation of Runx2 and receptor activator of nuclear factor κB ligand (RANKL), which colocalized in the calcified atherosclerotic lesions and were juxtaposed to infiltrated macrophages and osteoclast-like cells that are positively stained for an osteoclast marker, tartrate-resistant acid phosphatase. Mechanistic studies using RNA interference, a luciferase reporter system, chromatin immunoprecipitation, and electrophoretic mobility shift assays indicated that Runx2 regulated the expression of RANKL via a direct binding to the 5'-flanking region of the RANKL. Functional characterization revealed that RANKL did not induce VSMC calcification, nor was RANKL required for oxidative stress-induced VSMC calcification. Using a coculture system, we demonstrated that VSMC-expressed RANKL induced migration as well as differentiation of bone marrow-derived macrophages into multinucleated, tartrate-resistant acid phosphatase-positive osteoclast-like cells. These effects were inhibited by the RANKL antagonist osteoprotegerin and with VSMC deficient in Runx2 or RANKL.
Conclusion: We demonstrate that Runx2 directly binds to the promoter and controls the expression of RANKL, which mediates the crosstalk between calcifying VSMC and migration and differentiation of macrophages into osteoclast-like cells in the atherosclerotic lesions. Our studies provide novel mechanistic insights into the regulation and function of VSMC-derived RANKL in the pathogenesis of atherosclerosis and vascular calcification.
Figures





Comment in
-
Vascular calcification: harder than it looks.Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1249-50. doi: 10.1161/ATVBAHA.111.227868. Arterioscler Thromb Vasc Biol. 2011. PMID: 21593455 Free PMC article. No abstract available.
References
-
- Demer LL. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation. 1991;83:2083–2093. - PubMed
-
- Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. - PubMed
-
- Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. - PubMed
-
- Canfield AE, Doherty MJ, Wood AC, Farrington C, Ashton B, Begum N, Harvey B, Poole A, Grant ME, Boot-Handford RP. Role of pericytes in vascular calcification: a review. Z Kardiol. 2000;89(Suppl 2):20–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

