Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8
- PMID: 21455219
- PMCID: PMC3130899
- DOI: 10.1038/cdd.2011.27
Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8
Abstract
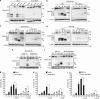
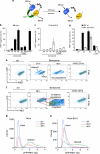
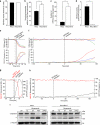
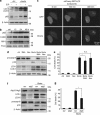
Antiapoptotic Bcl-2 family proteins are often highly expressed in chemotherapy-resistant cancers and impair mitochondrial outer membrane permeabilisation (MOMP), an important requirement for caspase activation via the intrinsic apoptosis pathway. Interestingly, although Bcl-2 overexpression in HeLa cervical cancer cells abrogated caspase processing in response to intrinsic apoptosis induction by staurosporine, tunicamycin or etoposide, residual caspase processing was observed following proteasome inhibition by bortezomib ([(1R)-3-methyl-1-({(2S)-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propanoyl}amino)butyl]boronic acid), epoxomicin (N-acetyl-N-methyl-lisoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-threoninamide) or MG-132 (N-(benzyloxycarbonyl)leucinylleucinylleucinal). Similar responses were found in Bcl-2-overexpressing H460 NSCLC cells and Bax/Bak-deficient mouse embyronic fibroblasts. Mild caspase processing resulted in low DEVDase activities, which were MOMP independent and persisted for long periods without evoking immediate cell death. Surprisingly, depletion of caspase-3 and experiments in caspase-7-depleted MCF-7-Bcl-2 cells indicated that the DEVDase activity did not originate from effector caspases. Instead, Fas-associated death domain (FADD)-dependent caspase-8 activation was the major contributor to the slow, incomplete substrate cleavage. Caspase-8 activation was independent of death ligands, but required the induction of autophagy and the presence of Atg5. Depletion of XIAP or addition of XIAP-antagonising peptides resulted in a switch towards efficient apoptosis execution, suggesting that the requirement for MOMP was bypassed by activating the caspase-8/caspase-3 axis. Combination treatments of proteasome inhibitors and XIAP antagonists therefore represent a promising strategy to eliminate highly resistant cancer cells, which overexpress antiapoptotic Bcl-2 family members.
Figures




References
-
- Carlucci A, Lignitto L, Feliciello A. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol. 2008;18:604–613. - PubMed
-
- Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. - PubMed
-
- Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21:871–877. - PubMed
-
- Colado E, Alvarez-Fernandez S, Maiso P, Martin-Sanchez J, Vidriales MB, Garayoa M, et al. The effect of the proteasome inhibitor bortezomib on acute myeloid leukemia cells and drug resistance associated with the CD34+ immature phenotype. Haematologica. 2008;93:57–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

