Targeting G-quadruplexes in gene promoters: a novel anticancer strategy?
- PMID: 21455236
- PMCID: PMC3119469
- DOI: 10.1038/nrd3428
Targeting G-quadruplexes in gene promoters: a novel anticancer strategy?
Abstract
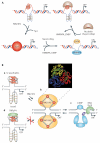
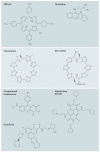
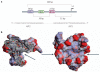
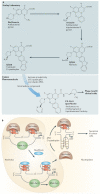
G-quadruplexes are four-stranded DNA structures that are over-represented in gene promoter regions and are viewed as emerging therapeutic targets in oncology, as transcriptional repression of oncogenes through stabilization of these structures could be a novel anticancer strategy. Many gene promoter G-quadruplexes have physicochemical properties and structural characteristics that might make them druggable, and their structural diversity suggests that a high degree of selectivity might be possible. Here, we describe the evidence for G-quadruplexes in gene promoters and discuss their potential as therapeutic targets, as well as progress in the development of strategies to harness this potential through intervention with small-molecule ligands.
Figures

References
-
- Kohn KW. Beyond DNA cross-linking: history and prospects of DNA-targeted cancer treatment — fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1996;56:5533–5546. - PubMed
-
- Roche VF. In: Foye’s Principles of Medicinal Chemistry. Lemke TL, Williams DA, Roche VF, Zito SW, editors. Lippincott Williams & Wilkins; Baltimore: 2008. pp. 1147–1192.
-
- Sen D, Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988;334:364–366. - PubMed
-
- Sundquist WI, Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989;342:825–829. - PubMed
-
- Zahler AM, Williamson JR, Cech TR, Prescott DM. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991;350:718–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

