Regulation of glioblastoma progression by cord blood stem cells is mediated by downregulation of cyclin D1
- PMID: 21455311
- PMCID: PMC3063796
- DOI: 10.1371/journal.pone.0018017
Regulation of glioblastoma progression by cord blood stem cells is mediated by downregulation of cyclin D1
Abstract
Background: The normal progression of the cell cycle requires sequential expression of cyclins. Rapid induction of cyclin D1 and its associated binding with cyclin-dependent kinases, in the presence or absence of mitogenic signals, often is considered a rate-limiting step during cell cycle progression through the G(1) phase.
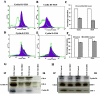
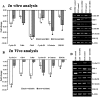
Methodology/principal findings: In the present study, human umbilical cord blood stem cells (hUCBSC) in co-cultures with glioblastoma cells (U251 and 5310) not only induced G(0)-G(1) phase arrest, but also reduced the number of cells at S and G(2)-M phases of cell cycle. Cell cycle regulatory proteins showed decreased expression levels upon treatment with hUCBSC as revealed by Western and FACS analyses. Inhibition of cyclin D1 activity by hUCBSC treatment is sufficient to abolish the expression levels of Cdk 4, Cdk 6, cyclin B1, β-Catenin levels. Our immuno precipitation experiments present evidence that, treatment of glioma cells with hUCBSC leads to the arrest of cell-cycle progression through inactivation of both cyclin D1/Cdk 4 and cyclin D1/Cdk 6 complexes. It is observed that hUCBSC, when co-cultured with glioma cells, caused an increased G(0)-G(1) phase despite the reduction of G(0)-G(1) regulatory proteins cyclin D1 and Cdk 4. We found that this reduction of G(0)-G(1) regulatory proteins, cyclin D1 and Cdk 4 may be in part compensated by the expression of cyclin E1, when co-cultured with hUCBSC. Co-localization experiments under in vivo conditions in nude mice brain xenografts with cyclin D1 and CD81 antibodies demonstrated, decreased expression of cyclin D1 in the presence of hUCBSC.
Conclusions/significance: This paper elucidates a model to regulate glioma cell cycle progression in which hUCBSC acts to control cyclin D1 induction and in concert its partner kinases, Cdk 4 and Cdk 6 by mediating cell cycle arrest at G(0)-G(1) phase.
Conflict of interest statement
Figures




References
-
- Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219–226. - PubMed
-
- Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. - PubMed
-
- Donato V, Papaleo A, Castrichino A, Banelli E, Giangaspero F, et al. Prognostic implication of clinical and pathologic features in patients with glioblastoma multiforme treated with concomitant radiation plus temozolomide. Tumori. 2007;93:248–256. - PubMed
-
- Kang SG, Kim JH, Nam DH, Park K. Clinical and radiological prognostic factors of anaplastic oligodendroglioma treated by combined therapy. Neurol Med Chir (Tokyo) 2005;45:232–238. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

